Ozonolysis
Of alkenes
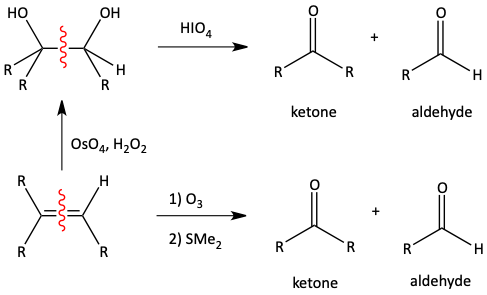
Ozonolysis is very similar to oxidation by KMnO4 in warm, concentrated solution. The difference is that if an aldehyde is formed, it is not further oxidized to a carboxylic acid. Notice that in this oxidation, two carbon- carbon bonds of the alkene turn into two carbon-oxygen bonds of the carbonyl.

The mechanism for this reaction does not really help us learn the reaction. If you are interested in it, you can look it up elsewhere. Ozone is a compound with three oxygen atoms (O3). Two of the three oxygen atoms are in the final product. The third oxygen atom ends up on the Me2S to make Me2SO.
Of alkynes
The reaction of an alkyne with ozone results in the same products as the full oxidation with KMnO4 under basic, hot conditions. The carbon-carbon triple bond is cleaved and each carbon atom is fully oxidized resulting in two carboxylic acids. Again, notice that in this oxidation, three carbon-carbon bonds of the alkyne turn into three carbon-oxygen bonds of the carboxylic acid.

Periodic acid cleavage of glycols
The C-C bond of a vicinal diol, a glycol, can be oxidatively cleaved with periodic acid (pronounced purr-eye-oh-dik-acid), HIO4 to make two carbonyl compounds. This is very similar to how alkenes are oxidatively cleaved in an ozonolysis reaction.
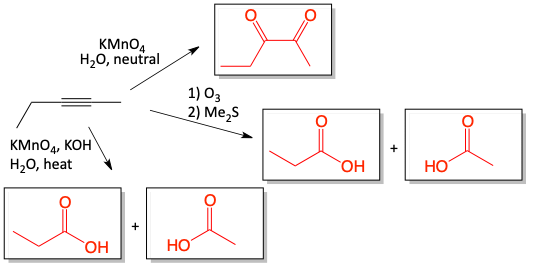
KMnO4 oxidation of alkynes
The same reaction can happen with the two pi-bonds of an alkyne. Since there are two pi-bonds, the dihydroxylation can happen twice, resulting in a species with four hydroxyl groups on it. This is unstable. Two water molecules are eliminated resulting in an α-diketone (alpha-diketone). Under neutral KMnO4 conditions, the α-diketone is the product.


The double dihydroxylation of the alkyne makes sense. Look closely how the two molecules of water are lost leaving only two oxygen atoms behind on neighboring carbon atoms.

Further oxidation with KMnO4
Of alkenes
If the alkene reaction with potassium permanganate is not conducted under dilute and cold conditions but is instead run under more basic conditions and warm and concentrated conditions, further oxidation can occur. When the glycol is further oxidized, the carbon-carbon sigma bond of the glycol is broken and the single carbon-oxygen bonds of the glycol turn into carbon-oxygen double bonds (an oxidation). KMnO4 is a pretty strong oxidizer. If an aldehyde is first made by this oxidation, it is further oxidized to a carboxylic acid.

and the further oxidation of the glycol with hot, concentrated KMnO4.

Overall, this reaction can be thought of as chopping the alkene in half and inserting two oxygen atoms. If the result is a ketone, nothing more happens to that carbon atom. If the result is an aldehyde, further oxidation to a carboxylic acid occurs.

Of alkynes
When KMnO4 reacts with an alkyne under more aggressive conditions, in the presence of hydroxide base and heat, the α-diketone is produced like under less aggressive neutral conditions, but it is then further, fully oxidized. The C-C bond between the two carbonyl groups is cleaved followed by further oxidation resulting in two carboxylic acids. With three C-O bonds, the carboxylic acid is fully oxidized and cannot be oxidized further.

and the further oxidation of the α-diketone with hot, concentrated KMnO4.

Overall, the cleavage can be thought of as. . .

Notice that in this oxidation, three carbon-carbon bonds of the alkyne turn into three carbon-oxygen bonds of the carboxylic acid.
4. Fill in the blank boxes.


Answers
4.