Ketones and Aldehydes from 1,3-Dithiane
Ketones and aldehydes can be synthesized in a nice, controlled fashion from 1,3-dithiane.
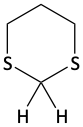
1,3-dithiane
The two protons shown on 1,3-dithiane are acidic. The dithiane can be easily deprotonated using butyl lithium (BuLi) as the base. Once deprotonated, the negatively charged carbon atom of the dithiane can act as the nucleophile in an SN2 reaction to alkylate the dithiane. This attaches one alkyl group to the dithiane making a monoalkylated dithiane (a thioacetal). If mercuric chloride (HgCl2) is added in acidic water, this alkylated dithiane would become an aldehyde. Alternatively, this monoalkylated dithiane can be deprotonated once again, followed by another SN2 reaction. This puts two alkyl groups on the dithiane. If this dialkylated dithiane is reacted with HgCl2, a ketone is produced.

5. Fill in the blank boxes.

Answers
5.


