Markovnikov hydration of alkyne
Alkynes, with their two pi-bonds, can also react in an oxymercuration-demercuration reaction. The reaction is very similar to that for an alkene with a twist at the end. Instead of mercuric acetate, the oxymercuration of alkynes usually involves mercuric sulfate and aqueous sulfuric acid. Like with alkenes, this results in the addition of water across a pi-bond resulting in a Markovnikov alcohol. Only one pi-bond of the triple bond reacted, so alkene remains. This vinyl alcohol is called an enol (pronounced “een-awl”). The “enol” name comes from this compound being an alkene (ene) and an alcohol (ol).

Enols tend to be unstable. Enols are in equilibrium with their isomer, the ketone. These isomers differ only in the location of one proton and a double bond. Such isomers are called tautomers (pronounced “taw-toe-mers”). The ketone and enol are called keto-enol tautomers. The transformation of one into another is called a keto-enol tautomerization. The process itself is called keto-enol tautomerism. In the equilibrium of the two, the keto form predominates. Take careful notice of the transformation. The hydrogen atom moves from the alcohol of the enol to the alpha carbon. The pi-bond of the alkene of the enol moves to make a carbonyl (a ketone).

Keto-enol tautomerization
Overall Reaction

The tautomerization is acid-catalyzed since sulfuric acid is used in the reaction. H+ is available because of the acid. To write the mechanism of the acid-catalyzed keto-enol tautomerization, protonate the enol at the alpha-carbon, draw resonance forms, and clean it up to make a ketone.
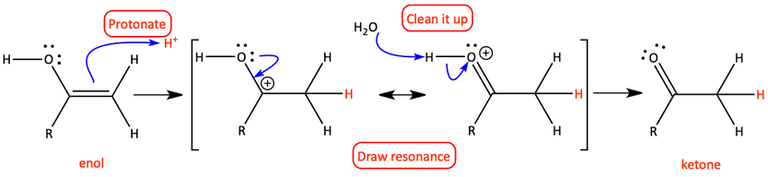
Acid-catalyzed keto-enol tautomerization
Hydroboration of alkyne
The hydroboration of an alkyne can lead to an anti-Markovnikov enol. Since alkynes contain two pi-bonds, two molecules of borane could add to it. A big, bulky, sterically hindered borane must be used in order to prevent this addition of two boranes. Usually, the borane used is di(sec-isoamyl)borane, commonly called disiamylborane. Instead of the small BH3 molecule, disiamylborane has two big, large, deer antler-like alkyl groups attached to it. There are five carbon atoms in each alkyl branch. Amyl is an old, common name for pentyl.
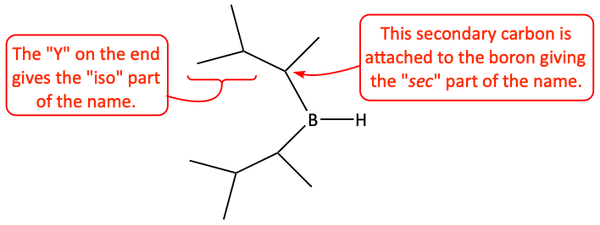
Disiamylborane reacts with an alkyne to make an anti-Markovnikov borane. This borane can be oxidized with peroxide and sodium hydroxide to make an anti-Markovnikov enol. This enol can tautomerize to the keto-form. In the case of a terminal alkyne, this keto-form is an aldehyde with a hydrogen attached to the carbonyl group.

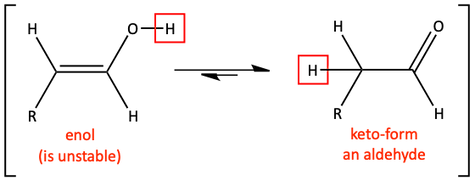
The mechanism of the base catalyzed keto-enol tautomerization is different than the mechanism was in acid. Bases deprotonate. So, the first step of this mechanism is the deprotonation of the enol at the alcohol position. This is pretty easy to remember because we are turning the alcohol portion of the enol into a carbonyl group. Once deprotonated, we draw resonance forms to get us closer to the keto product, then clean it up with some water to make the final aldehyde.

Base-catalyzed keto-enol tautomerization
3. Fill in the blank boxes.
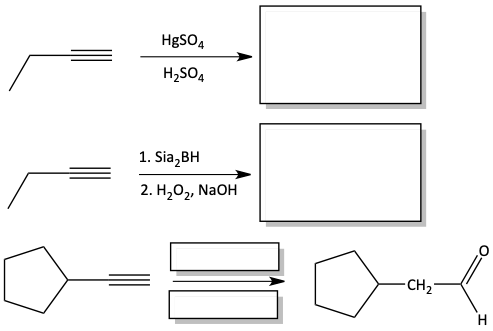
Answers
3.


