Conjugated Problems
Extra Problems
12. Draw the product when each of the following alkenes reacts with NBS?
a) cycloheptene
b) ethylbenzene
c) 1-pentene
d) 4-methylcyclopentene
13. Draw the structure of the major product which results when 2,3-dimethyl-1,3-butadiene is treated with HBr under warm, thermodynamic conditions.
14. Draw the products when the following pairs of reagents undergo a Diels-Alder reaction.

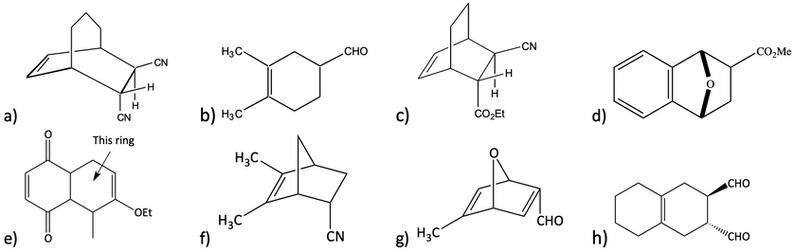
15. What diene and dienophile would react to give the product below?
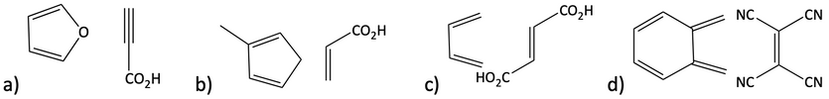
16. a) Draw the products when the following pairs of reagents undergo a Diels-Alder reaction. Sometimes, the reactants might need to be redrawn in a better orientation so you can better see how the reaction would proceed. b) Label each reagent for each reaction as either the diene or the dienophile.

17. What is A, B, and C in the following reaction sequence?

18. The pentadienyl anion, H2C=CH-CH=CH-CH2:-, has its lone pair of electrons delocalized over three carbon atoms.
a) Use resonance forms to show which three carbon atoms contain part of the lone pair of electrons.
b) Draw the molecular orbital diagram of the pentadienyl system in order of increasing energy. Label them π1, π2, π3, π4*, π5*. Fill in the pi electrons for each molecular orbital for this system in its ground state.
c) Sketch the molecular orbital for each energy level (π1, π2, π3, π4*, π5*).
d) Label the HOMO, LUMO, and draw the nonbonding line.
e) Explain how your molecular orbital diagram agrees or disagrees with the resonance picture showing delocalization of the lone pair of electrons onto three carbon atoms.
19. Draw the product(s) of the following reactions.

20. Show the step-by-step mechanism of the following reaction sequence.

21. Show the step-by-step mechanism of the following reaction sequence.

22. Show how you would synthesize the following compounds. You do not need to show mechanisms.

23. The following SN1, solvolysis, reaction results in two products. Show the mechanism for their formation.

24. The following free-radical bromination results in two different products. Draw those products and show the mechanism for their formation.

25. Draw good, reasonable resonance structures for the following intermediates.

26. Draw the product(s) of the following reactions.
27. a) Show how you would make 1,3-cyclopentadiene from cyclopentane.
b) In the lab, if you analyzed a flask full of 1,3-cyclopentadiene the day after you synthesized it, you would find the flask no longer contained 1,3-cyclopentadiene, but another compound. The 1,3-cyclopentadiene would react with itself via a Diels-Alder reaction. Show the mechanism for this reaction, and draw the structure of the new compound in the flask.
