Aromatic Addition Reactions
Aromatic Addition Reactions
A benzene ring has three pi bonds in it. These pi bonds are usually quite unreactive because it takes all three pi bonds to make the benzene ring a very stable aromatic ring. But, sometimes under fairly harsh conditions, a molecule can add across one of the pi bonds. Once one pi bond reacts, the aromaticity is broken. This allows the remaining two pi bonds to react rather easily. Under enough heat and pressure, or under ultraviolet light, the pi bond of benzene can react with Cl2 to be chlorinated. Once one pi bond is chlorinated, the other two pi bonds are chlorinated to make benzene hexachloride.
Chlorination of benzene

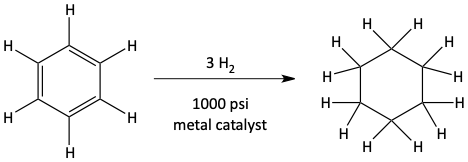
The pi bonds of benzene can be hydrogenated. Benzene can react with H2 and a metal catalyst under high pressure to become fully hydrogenated making cyclohexane.
Hydrogenation of benzene
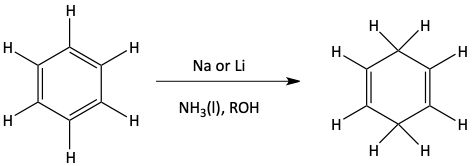
Birch Reduction
In the previous section we saw how benzene was fully hydrogenated with all three pi bonds reacting with hydrogen. It is possible for only two hydrogen atoms to be added to a benzene ring in a Birch reduction. If a benzene ring reacts with sodium and ammonia, the benzene can form a cyclohexa-1,4-diene.
The Birch reduction is of course a reduction. Reductions need electrons. It would be nice if we could simply buy a bottle of electrons. Since we cannot, we need to make some. The reaction of sodium and ammonia is a good way to make a beaker full of electrons.
For this reaction, sodium or lithium metal is dissolved in liquid ammonia. Ammonia has a boiling point of -33°C. It is a gas at room temperature. In order to turn the gaseous ammonia into a liquid solvent, it is cooled in a special chamber using dry ice. Once the ammonia is a liquid, pieces of solid sodium metal are added. Once enough sodium is added, it turns a beautiful blue color. This blue color means we have electrons trapped in the ammonia solvent. These are called “solvated electrons” or “ammoniated electrons”.

How does this work? Sodium metal is an alkali metal. It wants to give up one electron to become Na+. This is the source of the electrons. Ammonia has the perfect molecular shape to hold these electrons. Ammonia’s trigonal pyramidal shape forms a pocket with the positively charged hydrogen atoms that can hold a negatively charged electron. These freed electrons can be thought of as “electrons in a bottle”.

A negatively charged electron from the solvated electrons attacks the benzene ring. The electrons in the ring flow around to make a negatively charged carbanion as far away from the originally attacking electron as possible. The carbanion grabs a proton from the solvent.
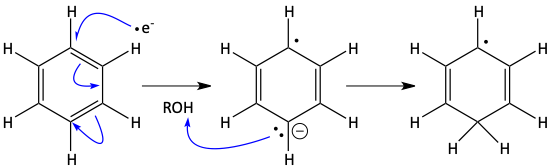
Another electron from the beaker full of electrons comes in to join its unpaired electron friend that is already on the ring forming a negatively charged carbanion. Once the carbanion is formed, it grabs a proton from the solvent making the cyclohexa-1,4-diene.

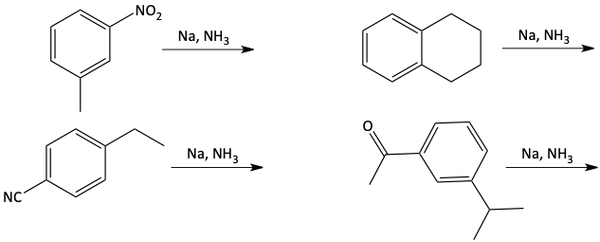
The two protons added to the benzene in a Birch reduction are on opposite sides of the ring. These two protons add where the negatively charged carbanions are formed. The best place for carbanions to form on a benzene ring would be where electron-withdrawing groups are attached like carbonyl, nitro, or cyano groups. Notice the hydrogen atom adds to the carbon atom of the benzene ring with the electron-withdrawing group attached.
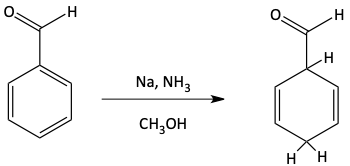
Carbanions would not want to form next to electron donating groups. So, the two hydrogen atoms added in a Birch reduction are not next to electron donating groups.

17. Draw the products of the following reactions.
Answers
17.

