Nucleophilic Aromatic Substitution
Nucleophilic aromatic substitution
We are going to switch gears a bit. We have been looking at many electrophilic aromatic substitutions where the pi bonds of a benzene ring attack a positively charged species. Now, we are going to look at nucleophilic aromatic substitutions, where a negatively charged species attacks our benzene ring. We have seen nucleophilic substitutions before. For instance, the SN2 mechanism is a nucleophilic substitution. We need a good attacking nucleophile with a large amount of electron density that attacks a substrate with a good leaving group.

This type of reaction normally does not work if the substrate is a halobenzene.

In order to get a halobenzene to do a nucleophilic substitution, we need to use a very strong nucleophile or make the benzene ring more reactive to the attack.
There are two main ways these substitutions occur. One is the Addition-Elimination mechanism and the other is the Benzyne mechanism.
Addition-Elimination (SNAr)
One way to make a nucleophilic aromatic substitution occur on a benzene ring is to make the benzene ring very electron poor so the negative nucleophile will want to attack it. The benzene ring is electron poor when electron-withdrawing groups like nitro, cyano, or carbonyl groups are attached. The first part of the Addition-Elimination mechanism (also called an SNAr mechanism) is obviously an addition. An electron rich nucleophile, in this case hydroxide (HO-), attacks and adds to the benzene ring. It attacks the benzene ring where the nucleophile is attached. The electrons of the benzene ring are pushed over and “stored” next to the electron withdrawing nitro group. Resonance forms can be drawn to spread out this negative charge, helping it be more stable. Look carefully at the resonance forms. Notice when the electron-withdrawing nitro groups are in the ortho and para sites, they are right next to the negative lone pair of electrons. Electron withdrawing groups at the ortho and para positions are much better than if they are at the meta positions.
The addition

After the addition, the elimination part of the Addition-Elimination mechanism must occur. The lone pair of electrons that had been “stored” fall back to remake the benzene ring and kick off the halide leaving group.
The elimination

If the nucleophile is ammonia, NH3, an aniline is formed. Notice that the final product is a benzene with an NH2 attached, not -NH3. One proton is removed with another molecule of ammonia in order to make the neutral aniline compound.

Sometimes, even if the leaving group is not a great leaving group, the reaction can still proceed.
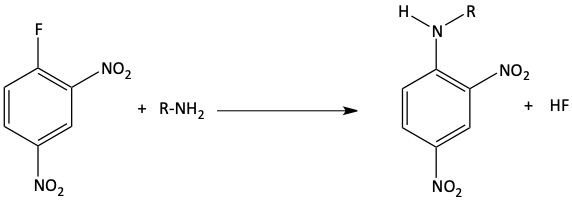
13. Which of the following aryl halides would undergo an addition-elimination reaction the best?
a)

b)

c)

d)

Benzyne
The other mechanism for nucleophilic aromatic substitutions is the benzyne mechanism. In the benzyne mechanism, the electron-withdrawing groups are usually absent on the benzene. Therefore, our benzene ring is not particularly electron poor. The only way to get the nucleophile to attack these benznenes is to use nucleophiles that are very electron rich and use harsh conditions, often heating the reactions to pretty high temperatures. Chlorobenzene can react with the fairly aggressive hydroxide nucleophile, but the reaction must take place at 350°C.

Sodium amide (NaNH2) is an even more aggressive nucleophile. The nitrogen atom likes the negative charge even less than the oxygen atom of the hydroxide. Sodium amide can perform the aromatic nucleophilic substitution at lower temperatures.

The mechanism for these reactions is called the Benzyne mechanism because it involves a benzene with a triple bond (alkyne) in it. In the Benzyne mechanism, the aggressive nucleophile performs an E2 type of elimination. It pulls off a proton beta to the leaving group. The electrons it leaves behind fall into the benzene ring forming a triple bond, the benzyne, as the leaving group leaves.

The aggressive nucleophile can then attack either side of the benzyne triple bond. It attacks and one pi bond from the triple bond goes onto the ring to form a carbanion. The carbanion is then protonated. Notice how either side of the benzyne triple bond can be attacked leading to a mixture of products that have the nucleophile in adjoining sites like ortho & meta or meta & para like this example.

14. Classify the following reactions as a benzyne aromatic substitution, an addition-elimination substitution, or neither.

Gilman Reagents
One other way to do a nucleophilic substitution on an aromatic halide with a carbon group is to use a Gilman reagent. A Gilman reagent is a lithium organocuprate. It is so named because it contains lithium, organic groups, and copper. Many different halide compounds (alkyl, vinyl, or aryl) can be used. The halide can be converted into an organolithium reagent by the addition of two equivalents of lithium metal. The organolithium reagent can be converted into a Gilman reagent with cuprous iodide, CuI.
Halide to organolithium
Organolithium to Gilman reagent

The R groups in the Gilman reagent are attached to the positively charged copper and lithium ions. The R groups are therefore negatively charged and are nucleophilic. They can perform substitution reactions with alkyl and even aryl halides!

Below are some examples of how this Gilman reagent coupling can be used. Notice how a carbon-carbon bond is formed in each of these. This is a little unusual compared to the previous ways we have learned to make carbon-carbon bonds. This substitution can occur on a double bond and even a benzene ring.
The substitution can occur on an alkyl halide.
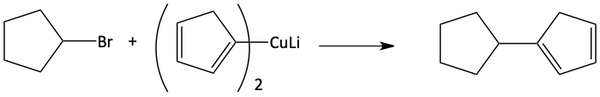
The substitution can occur on an aryl halide.

The substitution can occur on a vinyl halide.

The substitution can occur on an acid chloride.

15. Give the products of the following reactions.
a)
b)
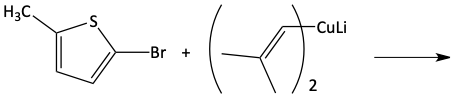
16. Give the Gilman reagent used in the following reactions.
a)

b)

Answers
13. b It has the most electron withdrawing groups (-NO2) in the ortho and para positions.
14.

15.
a)
b)

16.
a)

b)

