Introduction
Aromatic rings like benzene are very stable. Therefore, they do not react easily. They will react with a very strong electrophile. In this chapter, we will see several reactions that occur on aromatic rings. These are very important reactions as aromatic rings are found in many natural products and medicines.
Electrophilic Aromatic Substitution
Aromatic rings usually do not react unless they are in the presence of a very positively charged species, an electrophile. But, if an electrophile has a strong enough positive charge, it can coax the negatively charged pi electrons of the aromatic ring to attack it. When the pi bond attacks the electrophile, the electrophile attaches to the benzene ring, and the ring loses its conjugation. It forms a non-aromatic species called a sigma complex. A base cleans up the reaction by pulling off a proton with the electrons in its bond reforming the pi bond of the aromatic ring. Overall, a hydrogen atom is replaced with an electrophile.

Let’s look at a few of these reactions. You should immediately memorize these next few reactions. We will use them often.
Bromination of Benzene
We learned previously that pi bonds can be brominated using Br2 to make anti-dibrominated compounds. But, we find that benzene cannot be brominated the same way. The stability of the aromatic benzene ring precludes that.

A stronger electrophile is needed in order to brominate benzene or other aromatic compounds. This is done by adding the Lewis acid, ferric bromide (FeBr3), to bromine, Br2. Br2 donates a lone pair of electrons to the FeBr3 Lewis acid. This makes the bromine positively charged and an electrophile.

This is a strong enough electrophile that the aromatic benzene ring will now donate electrons from a pi bond to it. We will not worry about the mechanism of this reaction, but you should memorize it.

Chlorination of Benzene
The chlorination of benzene is similar to the bromination of benzene. On its own, chlorine is not a strong enough electrophile to react with an aromatic ring. A Lewis acid is needed. For the chlorination reaction, the Lewis acid is usually aluminum trichloride, AlCl3. Memorize this reaction.

Iodination of Benzene
The iodination of benzene occurs in the presence of iodine and nitric acid.

Iodine, I2, is also not a strong enough electrophile on its own. Typically, nitric acid, HNO3, is added to iodine to make the electrophilic iodonium ion.

Once iodonium is produced, the iodination of an aromatic compound can occur. Memorize this reaction.
Nitration of Benzene
Benzene is nitrated in the presence of nitric and sulfuric acid.

The mechanism to produce the electrophile in this reaction is interesting. When nitric acid and sulfuric acid react with each other, sulfuric acid acts like an acid and donated a proton while nitric acid acts like a base. Once the nitric acid is protonated, water leaves giving a nitronium ion. This nitronium ion is an excellent electrophile and can act with benzene producing a nitrobenzene.

Sulfonation and Desulfonation of Benzene
This reaction is fun because it uses fuming sulfuric acid which just sounds menacing. Fuming sulfuric acid is sulfuric acid with sulfur trioxide gas dissolved in it. In the presence of fuming sulfuric acid, benzene can be sulfonated.

Sulfonated benzene can be desulfonated when it is heated with water and acid.

1. Write the reagents used to perform the following transformations.
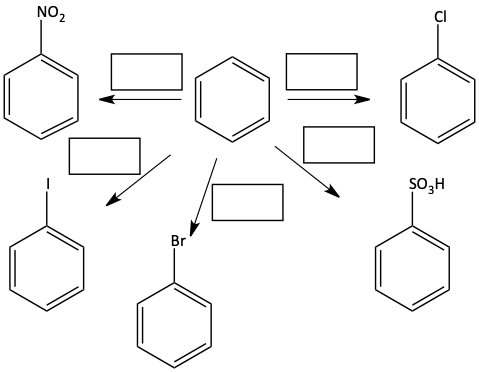
Activating Groups
Alkyl groups
Activated
Let’s look at the nitration of benzene compared to the nitration of toluene. Toluene is methyl benzene. We find that the nitration of toluene occurs faster than it does on benzene, 25 times faster. The methyl group makes the reaction go faster. We say that the methyl group, as well as other alkyl groups, is an activating group. Activating groups make aromatic reactions proceed faster.

Nitration of benzene

Nitration of toluene (occurs 25 times faster)
Ortho/Para Directing
When toluene is nitrated, the nitro group can go on ortho, meta, or para positions. If the process was totally random, we would expect ortho:meta:para isomers in a 2:2:1 ratio.

We find that much more of the ortho and para isomers are made with very little of the meta isomer being formed. We say that the methyl group, like other alkyl groups, is ortho-para directing.

The reason methyl and other alkyl groups are activating (making the reaction go faster) and ortho-para directing can be explained by looking at the mechanism of the nitration. When benzene reacts with the nitronium ion, the sigma complexes are all secondary cations.

When toluene is nitrated, we get sigma complexes that are both tertiary and secondary carbocations. Because tertiary carbocations are lower in energy (more stable) than secondary carbocations, reactions that make tertiary carbocations proceed faster than those that only make secondary carbocations. This is why the toluene reactions are activated. Notice that only the nitration at the ortho and para sites produce tertiary carbocation sigma complexes. This is why methyl and other alkyl groups are ortho-para directing.
Ortho attack

Nitration at ortho site gives tertiary carbocation
Meta attack

Nitration at meta site gives only secondary carbocations
Para attack

Nitration at para site gives tertiary carbocation
Alkoxy and amino groups
Alkoxy (-OR) and amine (-NR2) groups are even more activating than alkyl groups. The nitration of anisole (methoxy benzene) reacts 10,000 times faster than the nitration of benzene.

This fast nitration with alkoxy or amino groups is because carbocations that are formed in these sigma complexes are further stabilized through resonance. Also, when the nitration occurs at the ortho or para sites, this resonance-stabilized carbocation is formed but when the attack is at the meta site, no very good resonance stabilized sigma complexes are formed. For this reason, alkoxy and amino groups are ortho-para directing.

Ortho attack

Meta attack

Para attack

Alkoxy and amino groups are such excellent activating groups, that anisole and aniline can be brominated with bromine. The reaction doesn’t stop after one bromination. But, all of the ortho and para positions can be brominated.

Bromination of anisole

Bromination of aniline
The following chart shows the ranking of the activity of activating groups. The more electron donating the group, the more activating it is.

2. Draw the products of the following reactions.
a)

b)

c)

Deactivating Groups
Electron-donating groups are activating and ortho-para directing. If a group on an aromatic ring is an electron-withdrawing group, it has the opposite effect. Because electron-withdrawing groups suck electron density out of the aromatic ring, the ring is much slower to react. Therefore, an electron-withdrawing group slows down electrophilic aromatic reactions. A nitro group is an electron-withdrawing group. It is deactivating. While electron-donating activating groups make the ortho-para sites more reactive, electron-withdrawing deactivating groups make the ortho-para sites less reactive. Because the ortho-para sites are less reactive, we get more of the meta product. Deactivating groups are meta directors.

The nitration of nitrobenzene is 100,000 times slower than the nitration of benzene. It is deactivated. The nitration of nitrobenzene gives a majority of the meta product.

The nitration of nitrobenzene is 100,000 times slower than the nitration of benzene. It is deactivated. The nitration of nitrobenzene gives a majority of the meta product.

If we analyze the mechanism of electrophilic substitution on nitrobenzene, we can see why ortho and para substitution is less desirable. When ortho or para attacks happen, we get the bad situation where two positive charges next to each other.
Ortho attack

Meta attack

Para attack

Carbonyl groups are also meta-directing deactivating groups. This is because the carbon atom of a carbonyl has a partially positive charge.

In summary, electron withdrawing, meta-directing, deactivating groups all carry at least a partial positive charge. The more positive the group is, the more deactivating it is.

Halide Group
Because halides are pretty electronegative, they suck electron density out of an aromatic ring. This causes the aromatic ring to react slowly in an aromatic electrophilic substitution reaction. The halide is therefore a deactivating group. But, halides are a little different than other deactivating groups. Most deactivating groups like carbonyl or nitro groups are meta-directing. Halides, because they have lone-pairs of electrons on them, can help stabilize a carbocation through resonance much like alkoxy (oxygen lone pair) or amino (nitrogen lone pair) groups did. Therefore, halides are not meta-directing, but they are ortho-para directing like electron-donating, activating groups are.
The nitration of chlorobenzene leads to mostly the ortho-para products because chloride is an o,p-director (even though the reaction is slow because chloride deactivates the ring).

Summary

Summary of the Directing Effects of Substituents
We have seen previously that the amino group of aniline (aminobenzene) is a very activating, ortho-para, director. In fact, the bromination of aniline happens quite fast at all of the ortho and para sites giving 2,4,6-tribromoaniline.

Interestingly, the nitration of aniline is quite different. When aniline reacts with nitric and sulfuric acid, the reaction is slow and gives primarily meta-nitroaniline. The reason this occurs is the nitrogen atom of the amino group of aniline is basic. It is protonated under the acidic conditions of the nitration to become a quaternary amino salt, -NH3+, a deactivating, meta-directing group.

3. Draw the products of the following reactions.
a)
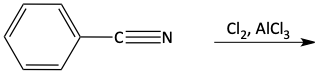
b)

c)


d)
Disubstituted benzenes
A disubstituted benzene is when two groups are on a benzene. When both groups are activating, the benzene ring is activated. When both groups are deactivating, the ring is deactivated. When one group is activating and one is deactivating, it is difficult to tell.

When an electrophilic aromatic reaction occurs, analyze how each group directs the substitution. If the two groups agree where the substitution should occur then the substitution occurs there. If a site is sterically hindered, the substitution may not occur at that site. For m-ethyl methylbenzene, both alkyl groups are o, p-directing. Each alkyl group directs to the same locations. The red and blue arrows point to the same sites. The site between the two alkyl groups is a little crowded, so the substitution does not happen much there.


For p-methyl benzaldehyde, the methyl group is ortho directing. The carbonyl group is meta directing. These agree for the location of the substitution.

For o-xylene, each methyl group is o,p-directing, but they direct to different locations. A mixture of products is formed.

For m-nitroanisole, the methoxy is o,p-directing but the nitro is meta directing. These point to different locations. When there is a disagreement in locations, the group that is more activating wins because the reactions happen faster at those sites. In this case, methoxy (OCH3) wins. Again, the site in between the two groups is pretty crowded, so the reaction does not happen there as much.

The strength of directing groups was summarized in the previous section. Pi donors (-OH, -OR, -NR2) are more activating than sigma donors (alkyl groups). Halides are a little deactivating, but are not as deactivating as fully meta-directing deactivating groups like –NO2, -CN, and carbonyls). Go back and study the directing group summary figure in the previous section.
4. Draw the products of the following reactions.
a)


b)
Answers
1.

2.
a)

b)
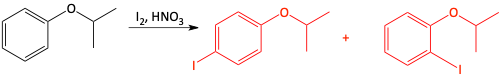
c)

3.
a)

b)

d)

c)

4.
a)

b)


