Friedel-Crafts
Friedel-Crafts alkylation
A Friedel-Crafts alkylation is a reaction where an alkyl group is added to an aromatic ring. In a Friedel-Crafts alkylation, an alkyl halide reacts with a Lewis acid like AlCl3 or FeBr3.

Below is an example Friedel-Crafts reaction.

In the mechanism of the Friedel-Crafts reaction, the alkyl halide reacts with the Lewis acid to form a carbocation on the alkyl group. This positively charged alkyl group is a strong electrophile. Since a carbocation is formed, the Friedel-Crafts reaction works best on secondary or tertiary alkyl groups since they make the most stable carbocations.

In the following example of a Friedel-Crafts reaction, the chloride of tert-butyl chloride is removed by the Lewis acid aluminum trichloride, AlCl3, forming a stable tertiary carbocation.

A pi bond of the benzene ring attacks the electrophilic carbocation forming a sigma complex.

The sigma complex is deprotonated. The electrons return to reform a pi bond reforming the aromatic benzene ring. The final product is an alkyl benzene.

Even though secondary and tertiary carbocations are more stable, it is possible for a primary alkyl halide to do a Friedel-Crafts reaction even though primary carbocations do not want to form!

If a primary carbocation is going to be formed, the attack by the benzene happens at the same time as the chloride leaves.
In the following reaction, a primary ethyl group is added to benzene by the reaction of ethyl chloride with AlCl3.

Limitations of Friedel-Crafts alkylation
There are three main limitations to the Friedel-Crafts alkylation. 1. Deactivated rings do not work. 2. Carbocations can rearrange. 3. Polyalkylations are possible.
1. Deactivated rings do not work. In order for a benzene ring to do a Friedel-Crafts alkylation, the ring needs a sufficient amount of electron density in it. Friedel-Crafts alkylations do not work on benzene rings that are more deactivated than halobenzenes. Benzene rings with nitro groups, cyano groups, or carbonyl groups attached will not work. For instance, nitrobenzene will not undergo a Friedel-Crafts alkylation.

This is something to consider in some syntheses. For instance, let’s say we wanted to make p-ethyl nitrobenzene from benzene. Two groups need added to the benzene, an ethyl and a nitro group.

The order in which we add these groups is important. In reaction 1, we put the nitro group on first. This deactivates the ring and the subsequent Friedel-Crafts alkylation to add the ethyl group will not work. In reaction 2, we put the ethyl group on first with a Friedel-Crafts alkylation. The ethyl group is activating and ortho/para directing. The subsequent nitration easily works giving a mixture of the ortho and para products.

2. Carbocations can rearrange. In a Friedel-Crafts alkylation, the halogen of an alkyl halide is removed with AlCl3 for form a carbocation. Any time a carbocation is formed, we need to watch out for rearrangements. For instance, in the following example, if we wanted to make propyl benzene, we might try to react benzene with propyl chloride and AlCl3.

This reaction does not work as intended. We find that propyl benzene is not made, but instead, isopropyl benzene is formed.

The reason isopropyl benzene is formed is the primary propyl cation rearranges. It does a 1,2-hydride shift to form a more stable secondary carbocation.

This secondary carbocation does the Friedel-Crafts alkylation with the benzene to form isopropyl benzene.

3. Polyalkylations are possible.
Because alkyl groups are activating groups, once one alkyl group is added to a benzene ring, it makes the benzene ring even more activated. The ring becomes even more reactive, so the ring can be alkylated again. Therefore, the ring can become di- and tri-alkylated.

5. List the three limitations usually associated with the Friedel-Crafts alkylation reaction.
Friedel-Crafts acylation
An acyl group is a carbonyl ketone or aldehyde group.

An acyl group can be added to a benzene ring through a Friedel-Crafts acylation reaction.
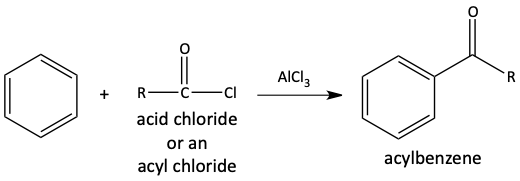
This is very similar to a Friedel-Crafts alkylation. A chloride is removed from an acid chloride with AlCl3 to make a carbocation acylium ion.

The acylium ion is a stable carbocation because it has a good resonance form. It is so stable; it does not rearrange.

The acylium ion reacts with the benzene ring through an electrophilic aromatic substitution to make an acylbenzene.

The acid chlorides used in Friedel-Crafts acylations can be synthesized from the appropriate carboxylic acid and thionyl chloride (SOCl2).

Let’s consider the three limitations of Friedel-Crafts alkylations and see how Friedel-Crafts acylations compare.
Limitations of Friedel-Crafts acylation
1. Deactivated rings do not work. Once again, if the ring is deactivated more than halobenzenes, the Friedel-Crafts reaction does not work. This is the same for alkylations and acylations. Therefore, Friedel-Crafts reactions do not work on nitro, cyano, or acylbenzenes.
2. Friedel-Crafts acylation carbocations do not rearrange. In the Friedel-Crafts acylation, a resonance stabilized acylium ion is formed. It is so stable; rearrangements do not occur like they can in an alkylation.
3. Friedel-Crafts acylations do not polyacylate. When benzene is alkylated using a Friedel-Crafts alkylation, the alkyl benzene product that is made is more activated than the original benzene. This is because alkyl groups are activating groups. In a Friedel-Crafts acylation, the acylbenzene product formed is deactivated since carbonyl acyl groups are deactivating groups. Therefore, only one acyl group attached to the benzene ring. They do not polyacylate.

Example of Friedel-Crafts Acylation
6. Why are Friedel-Crafts acylation reactions not prone to polyacylation?
Gatterman-Koch formylation
Consider if we wanted to make benzaldehyde from benzene using a Friedel-Crafts acylation. We would want to use a reagent called formyl chloride. A formyl group is an aldehyde group.

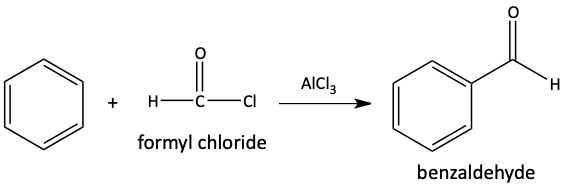
The problem with making benzaldehyde in this way is formyl chloride is unstable. We cannot buy a bottle of formyl chloride. Therefore, we must make formyl chloride when we need it. We make formyl chloride using carbon monoxide (CO) and hydrochloric acid (HCl). With the addition of the Lewis acid, AlCl3, a formyl cation is formed. Upon the reaction with benzene, benzaldehyde is formed. This reaction of making aromatic aldehydes is called a Gatterman-Koch formylation.

7. Draw the products of the following reactions.
a)

b)

c)

d)

Clemmensen Reduction
Acylbenzenes can be reduced to alkyl benzenes using the Clemmensen Reduction. This is a reduction because a carbonyl with two carbon-oxygen bonds is reduced to an alkyl group with zero carbon-oxygen bonds.

Clemmensen Reduction reagents are a zinc/mercury amalgam in the presence of aqueous hydrochloric acid.

Think back to the difficulty we had making propyl benzene in a Friedel-Crafts alkylation. Instead of propyl benzene, we made isopropyl benzene because the alkyl carbocation rearranged. A combination of Friedel-Crafts acylation followed by Clemmensen Reduction allows us to make propylbenzene.

The reason this combination works is the acylium ion formed in the Friedel-Crafts acylation does not rearrange. The reduction of the acyl group gives us the alkyl group. This is the primary way we can make alkylbenzenes with primary alkyl groups.
8. A chemist attempted to make propyl benzene by performing a Friedel-Crafts alkylation on benzene using propyl chloride and AlCl3. Unfortunately for the chemist, a rearrangement occurred and isopropyl benzene was made.

The chemist then decided to make propyl benzene from a Friedel-Crafts acylation followed by a Clemmensen Reduction. Show this synthesis and explain why this works better than the Friedel-Crafts alkylation did.
Answers
5.
1. They can polyalkylate because after the first alkyl group goes on the ring, it becomes even more reactive because alkyl groups are activating.
2. They do not work on deactivated rings.
3. They can rearrange because they have a carbocation intermediate.
6. Once the first acyl group goes onto the ring; the ring becomes deactivated because carbonyl-containing groups are electron withdrawing.
7.
a)

b)

c)

d)

8.

