Diels-Alder Reaction
Overview
Otto Diels and Kurt Alder were awarded the 1950 Nobel Prize in Chemistry for their work on a very important reaction. This reaction, the Diels-Alder reaction, is a [4+2] cycloaddition. A [4+2] cycloaddition means four atoms react with two atoms to make a ring. In the Diels-Alder reaction, a conjugated diene reacts with an alkene that is commonly called a dienophile (lover of the diene) to make a 6-membered ring.
Remember that six-membered rings do not have much ring strain. Therefore, six-membered rings are very common in nature. We find them in many natural products and medicines. Therefore, the Diels-Alder reaction is a very commonly used reaction by synthetic organic chemists.

Diels-Alder reaction, a [4+2] cycloaddition
The Diels-Alder reaction has a concerted mechanism. This means all of the bonds are broken and made simultaneously. We usually draw the diene and dienophile lined up and then draw three arrows “around the horn”.

The diene is usually electron rich. You will commonly find electron donating groups on the diene which push even more electron density into the conjugated diene. Electron donating groups that you will commonly see are alkyl groups, -OR, or –OH. Alkyl groups donate electron density through hyperconjugation and OR or OH donate electron density through resonance with their lone pairs of electrons. The dienophile prefers to be electron poor so it can accept the electrons from the electron rich diene. Therefore, the dienophile often has electron withdrawing groups on it. Good electron withdrawing groups are often carbonyl groups or cyano groups. They can pull electron density away from the dienophile through resonance.

s-cis conformation
Both double bonds of the diene need to point towards the dienophile in order for the Diels-Alder reaction to proceed. This conformation of the diene is called the s-cis conformation. Previously, we learned that we used the prefix cis when two groups were on the same side of something that cannot rotate, like a double bond or a ring. We used trans when two groups were on the opposite side of a double bond or a ring. In the case of this diene, the two double bonds are on the same side of a single bond, or sigma bond. This is a different situation than when we used cis/trans before because it is possible to rotate completely around a single bond. Therefore, we designate these conformations to describe whether the double bonds are on the same side of the single bond, s-cis, or opposite sides of the single bond, s-trans.

s-cis and s-trans conformation
The more easily the diene can get into an s-cis conformation, the more easily it can undergo a Diels-Alder reaction.

8. Rank the following dienes for their reactivity in a Diels-Alder reaction from most reactive (1) to least reactive (5).

Orbital overlap
Below are the MO diagrams for the simplest diene (1,3-butadiene) and the simplest dienophile (ethane). When a reaction, like the Diels-Alder reaction, takes place, one of the reactants usually donates some electrons from a filled molecular orbital into an empty molecular orbital of the other reactant. In this case, the electron rich diene will donate a pair of electrons from its HOMO (𝜋2) into the LUMO of the dienophile (𝜋2*).

The following diagram shows the overlap of the molecular orbitals of the HOMO of the diene with the LUMO of the dienophile. Notice how the orbitals overlap perfectly. The constructively overlapping orbitals are in phase with each other.

Orbital overlap for Diels-Alder reaction
Also, notice that the cyclohexene that is formed is not flat. It is puckered with an envelope shape.
Syn stereochemistry
If the dienophile has two groups on it that are cis, they end up cis on the cyclohexene ring formed. If the two groups are trans on the dienophile, they are trans on the cyclohexene ring. This is because the p-orbitals of the diene and dienophile must overlap and the mechanism for the reaction is concerted. There is no time for the groups to change their configuration.

Syn-stereochemistry for Diels-Alder reaction
9. Draw the products of the following Diels-Alder reactions.
a)

b)

c)

Endo rule
Similarly, if an electron-withdrawing group on the dienophile has a pi bond, it will overlap with the p-orbitals of carbon atoms C2 or C3 of the diene. This secondary overlap helps stabilize the transition state of the reaction. The result of this secondary overlap is the electron-withdrawing group ends up inside the pocket of the puckered envelope of the cyclohexene. This is called the Endo rule.
The groups that point into the puckered envelope fold of the cyclohexene are called the endo positions and those that point outside of the puckered fold are called the exo positions.

Endo vs. Exo positions
Let’s look at the following Diels-Alder reaction.

10. In the following Diels-Alder reactions, one hydrogen atom and one other group is missing in each of the final products. Draw in the missing hydrogen atoms and missing groups giving careful attention to the Endo Rule.
a)

b)

Diels-Alder regiochemistry
Finally, sometimes a decision needs to be made on the regiochemistry of the cyclohexene product. For instance, if 2-methoxy-1,3-butadiene reacts with cyanoethene, which product do we get? 1,3-addition or 1,4-addition? Here are the two possibilities.

The best way to analyze the regiochemistry is to investigate where the most negative site is on the electron rich diene. We draw resonance forms to push the electrons from the electron-donating group out onto the end of the diene.

Then, we use resonance forms to determine the most positive site on the electron poor dienophile. We push the electrons away from the alkene onto the electron-withdrawing group.

We then put the most negative site of the diene next to the most positive site of the dienophile and perform the Diels-Alder reaction. In this particular case, we see that the 1,4-addition product is made.

In the reaction of 1-methoxy-1,3-butadiene with cyanoethene, 1,2- or 1,3-addition are both possible. Which is the correct product? Here are the two possibilities.

Once again, we draw resonance forms to find the most negative site on the diene by pushing electrons from the methoxy electron-donating group to one of the edges of the diene.

We then once again find the most positive site on the electron poor dienophile by finding resonance forms by pushing electrons from the alkene onto the electron-withdrawing group.

We put the most negative site of the diene with the most positive site of the dienophile and perform the Diels-Alder reaction. The final product in this case is the 1,2-addition product. We find that 1,2 and 1,4 addition are common in Diels-Alder reactions, but the 1,3-addition product is not very common.
11. Draw the products of the following reactions giving careful attention to regiochemistry.
a)

b)

Answers
8.

9.
a)
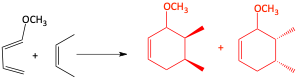
b)

c)

10.
a)

b)

11.
a)

b)





