Conjugation
Conjugation introduction
In this chapter, we are looking to deeply understand conjugated alkenes. Conjugated double bonds or conjugated alkenes are those double bonds that are separated by only one single bond. Conjugated double bonds have a double-single-double pattern of carbon bonds.

Conjugated double bonds are more stable, or lower in energy, than isolated double bonds. For example, 1,4-pentadiene, with isolated double bonds, has an energy of 57.4 kcal. The very similar, 1,3-pentadiene has an energy of 53.7 kcal. The only difference is 1,3-pentadiene has a conjugated pi system. This conjugation results in nearly 4 kcal of stability compared to the isolated double bonds.

This extra stability because of conjugation can be explained by looking at the pi-system molecular orbital (MO) diagrams. Let’s begin our investigation of pi-system MO diagrams by first looking at the simplest case of pi-bonding, ethene. The double bond of ethene consists of one sigma-bond made from two overlapping sp2 orbitals and one pi-bond with two overlapping p orbitals (in blue).

Now, let’s look only at the pi bond of ethene and ignore the sigma bonds. The atomic p orbitals can overlap constructively to make a pi bond or destructively to make an antibonding 𝜋*. The 𝜋 designation is used because the electron density lies above and below the internuclear axis.

We can describe the pi bonding for ethene using the following molecular orbital diagram.

Molecular orbital diagram for ethene
Each p atomic orbital contains one electron. When combined, the bond order is 1 for this pi system. There is only one pi bond formed.
Bond order = 1 full bond – 0 full antibonding MOs = 1
1. Circle the following molecules that are conjugated alkenes.

1,3-Butadiene
Butadiene is a simple, conjugated pi system.
1,3-butadiene
To make the two double bonds, four carbon p-orbitals overlap. Each of the p-orbitals contains one electron each. Since these four p atomic orbitals are overlapping, it makes four molecular orbitals in the MO diagram. Since there are always an equal number of bonding and antibonding molecular orbitals, butadiene has two bonding molecular orbitals and two antibonding molecular orbitals.
To determine the shapes of the molecular orbitals, we overlap the four p-orbitals. Notice that in the lowest energy molecular orbital, there are no nodes. All of the p-orbitals overlap making one pi molecular orbital that spreads over the entire four carbon atoms. This is labeled 𝜋1 in the MO diagram. There are two electrons in this orbital that are spread over the entire four carbon atoms.
The next higher energy molecular orbital, 𝜋2, has one node in it, right in the center. The electron density of 𝜋2 is located between carbon atoms C1-C2 and C3-C4. There are two electrons in this molecular orbital. This electron density is quite similar to the way we draw butadiene, with pi bonds drawn between carbons C1-C2 and C3-C4. Between carbon atoms C2-C3, there is destructive overlapping of the p-orbitals. But, overall, since there are two bonding sites and one antibonding site, the molecular orbital is bonding.
The electrons in 𝜋2 are the highest energy electrons in the molecule because they are in the highest occupied molecular orbital. This molecular orbital is given the acronym HOMO for Highest Occupied Molecular Orbital. These highest energy electrons are the most reactive electrons in butadiene.
The next higher molecular orbital is higher in energy than the four atomic p-orbitals. This molecular orbital, 𝜋3*, contains two nodes. This orbital has some overlapping p-orbitals between carbon atoms C2-C3, but it is destructively overlapping in two places, between carbon atoms C1-C2 and C3-C4. Overall, this is more antibonding than it is bonding. This molecular orbital does not have any electros in it because the four p-orbitals each only had one electron for a total of four electrons that can be used in the molecular orbitals. All four were used in 𝜋1 and 𝜋2. Since 𝜋3* is the lowest molecular orbital without electrons in it, it is labeled with the acronym LUMO for Lowest Unoccupied Molecular Orbital.
Finally, the highest molecular orbital of the conjugated pi system of butadiene is 𝜋4*. In 𝜋4*, all of the p-orbitals are out of phase with each other and destructively overlapping with each other. This gives three nodes for a totally antibonding orbital.

Molecular orbital diagram for 1,3-butadiene
The energy distance between the HOMO and LUMO molecular orbitals is of particular importance. This energy gap between the highest occupied molecular orbital and the lowest molecular orbital is called the HOMO-LUMO band gap or simply band gap. As the number of conjugated pi bonds increases in conjugated molecules, more molecular orbitals are needed to describe the conjugated system. This results in shrinking or smaller HOMO-LUMO band gaps energies.
2. Shade in the atomic p-orbitals to represent what would overlap to make the following molecular orbitals.
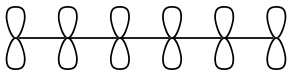
The HOMO of 1,3,5-hexatriene

The LUMO of 1,3,5-hexatriene

HOMO-LUMO band gap of conjugated compounds
For ethane, the HOMO-LUMO band gap is fairly large. In fact, the gap is of an energy that equals 175 nm of light. This is in the ultraviolet range. This is to say, if ultraviolet light of frequency 175 nm shines on ethane, ethane will absorb that light by promoting an electron from the HOMO to the LUMO. 1,3-butadiene, with more conjugation, has a lower HOMO-LUMO band gap. Ultraviolet light of 217 nm can promote an electron from its HOMO to its LUMO energy level. 1,3,5-hexadiene has an even smaller HOMO-LUMO band gap. It will absorb ultraviolet light with a wavelength of 258 nm. Notice, longer wavelength light equals lower energy.
All three of these compounds, ethane, 1,3-butadiene, and 1,3,5-hexatriene, all absorb light in the invisible, ultraviolet region. Because invisible light is being absorbed, all of these compounds are colorless. In order for a compound to have a visible color, the band gap needs to be sufficiently small to absorb wavelengths in the visible spectrum, between about 400-700 nm. The way organic molecules get this small of a band gap is to be very conjugated. Here are some examples of colorful pigments with their absorptions.

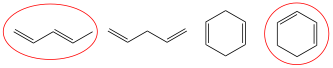
3. Circle the one molecule in the following grouping that absorbs the longest wavelength of light?

UV-Visible Spectroscopy
We have seen that conjugated molecules can absorb ultraviolet and visible light. The process of measuring the absorption of ultraviolet and visible light is called UV-Visible (UV-Vis) spectroscopy. A UV-Vis spectrometer is an instrument that can shine specific wavelengths of ultraviolet or visible light through a sample. The sample is usually dissolved in a solvent and placed in a cuvette. A cuvette is a 1 cm X 1 cm sample holder. Cheap ones are made out of plastic or glass. But, glass and plastic absorb ultraviolet light. So, good cuvettes are made out of quartz and can allow ultraviolet light pass through them. A detector measures the amount of each wavelength of light that passes through the sample. If the sample begins to absorb the light, less passes through to the detector, and a peak is made. It shows an absorbance.

Simple concept for UV-Visible spectrometer
A UV-Vis spectrum usually plots absorbance vs. wavelength of light that passes through it in nm. Let’s pretend that we are analyzing blue juice. The UV-Vis instrument begins scanning from 200 nm (the ultraviolet region) to 400 nm (violet light) on up to 800 nm (red light). Below is the UV-Vis spectrum. It shows that no light is absorbed between 200-500 nm (ultraviolet through purple). Then, the sample begins absorbing light between 500-700 nm. The light around 600 nm is the greatest absorbance with the highest spot at 605 nm. 605 nm is therefore called the λmax (lambda max). This is the wavelength (λ) with the maximum absorbance. Since the juice absorbs light in the yellow-orange part of the spectrum, it looks blue.

Even though a HOMO-LUMO gap is a very specific energy, UV-Vis peaks tend to be broad. This is because there are different rotational and vibrational states and energies for the molecule. This causes the energies of the HOMO and LUMO molecular orbitals to vary some.
Solved problem:
Given this UV-Vis spectrum, what is the energy of the HOMO-LUMO band gap for this blue juice?
The λmax for the blue juice is 605 nm. This wavelength corresponds to a specific energy. We know that the energy of light is proportional to its frequency.
E = hν
We therefore need to know the frequency of this light given its wavelength. We know wavelength and frequency are related by the speed of light.
c = λν
Using this equation, we solve for frequency. The units should be in m/s for the speed of light and in m for the wavelength.

With the frequency, the energy can now be calculated. Plank’s constant, h, is 6.63E-34 Js.

This is the approximate energy of the HOMO-LUMO band gap.
Answers
1.
2.

3.

