Solved Spectroscopy Problem
Solved Problem
Let’s work through identifying the structure of an unknown compound by using spectroscopic data. The mass spectrum had an M+ of 88 with no significant M+2 peak. This even M+ would indicate our unknown does not have an odd number of nitrogen atoms. No M+2 peak means we do not have Cl, Br, or S. The IR spectrum is then taken.

The peak below 3000 indicated sp3 C-H. The strong 1735 peak indicates a carbonyl. A normal carbonyl is around 1710. Since our number is high, in the 1730’s, it indicates the carbonyl of an ester or a ring-strained ketone. The other two strong peaks at 1100 and 1200 in the fingerprint region bolster the ester guess. If we have an ester, we have at least one carbon atom and two oxygen atoms in our molecule. CO2 weighs 44. This leaves us with 44 more in our mass to get to 88. This remaining 44 of the weight could be three carbon atoms (36) and eight hydrogen atoms (8). This would give a molecular formula for an ester of C4H8O2. We can calculate degrees of unsaturation:

This formula gives us one degree of unsaturation, which makes sense for a carbonyl of an ester. If we did not get a whole number with this calculation, we would know that our formula was incorrect.
So far, we know we have. . .

This is in the middle of the molecule (we haven’t drawn the ends yet). We still have three more C atoms and eight H atoms still to place.
Now, let’s turn to the NMR spectra. It is usually best to begin with the 13C NMR spectrum and DEPT to begin to understand the carbon framework of our molecule.

The regular 13C NMR (DEPT-0) shows we have four different types of carbon atoms (labeled A-D). Peak A is above 100 ppm, so it indicates a carbonyl carbon, which is good if we have an ester. It does not show up in the DEPT-45, so it does not have a hydrogen atom on it (like an aldehyde would). This is again consistent with our ester prediction. Carbon peaks B-D represent three different carbon atoms. They are all below 50 ppm, so they are “regular” carbon atoms. B-D all show up in the DEPT-45, so they all have hydrogen atoms on them. None of them show up in the DEPT-90, so none of them are CH carbon atoms. In the DEPT-135, we see that B points down (CH2), C and D point up (CH or CH3) but we know they are not CH from the DEPT-90. Therefore, we know that carbons C and D are CH3, methyl, carbon atoms.
Let’s pause and consider if the groups are on the inside of the molecule or on the outside ends. Carbons C and D are on the outside, ends, of the molecule because methyl groups, -CH3, only have one more bond going to them. Carbon B is a methylene, -CH2-, group. It is on the inside of our molecule as it has two more bonds going to it. If we put the pieces together, we have two possibilities.
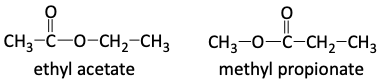
Now, let’s turn to the 1H NMR spectrum to finish up our analysis. As we do so, let us remember that we are looking for three proton groups we have identified so far, two methyl –CH3 groups and one methylene –CH2– group. Let’s be careful to look for these groups and not unnecessarily complicate things. For example, if we cannot tell if a group integrates to 1H or 2H, we will guess it is 2H since we have a CH2 and not a CH.

For the 1H NMR spectrum, we see that the quartet, E, integrates to 2H (CH2) and since it is a quartet, is next to one of the CH3 groups we identified earlier. The singlet, F, integrates to 3H (CH3) and since it is a singlet, is next to a carbon atom with no hydrogen atoms on it. The triplet, G, integrates to 3H (CH3) and since it is a triplet, is next to the CH2 group (E). This shows us we were on the right track, since we have a triplet-quartet ethyl pattern (-CH2CH3) and a lone methyl group (-CH3). But, which of our two possibilities is correct? Is it ethyl acetate or methyl propionate?
We turn to the chemical shifts to determine which structure is correct. Notice, E (CH2) is farthest to the left or downfield. These protons are probably next to something special, like the oxygen atom. The singlet F, the lone CH3, is around 2 ppm, which indicates it is next door to the carbonyl. The methyl, G, is farthest upfield and is far from any special group. This shows us that ethyl acetate is the correct structure. If the unknown was instead methyl propionate, groups E and F would be swapped in our 1H NMR spectrum.

