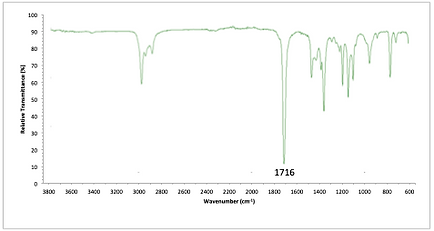
NMR Problems
Extra Problems
12. For each molecule, indicate the number of unique proton groups and carbon atoms on the lines provided (i.e., how many you would see in the NMR).
a)
b)

c)
d)

e)

f)

g)

h)

i)

j) bromopropane
k) 1-bromo-1,1-dimethylethane
13. Identify the approximate chemical shifts (in ppm) of the protons in the following compounds.
a) CH3CH2CHO
b) Ph-CH3
c) (CH3)3N
d) H2C=CH-Ph
e) CH3CH2CO2H
f) CH3OCH3
g) CH3CH2Br
14. Identify the chemical shift range (0-50, 50-100, 100-150, 150-200 ppm) of the carbons in the following compounds.
a) CH3CH2CHO
b)

c)
d)

e)

f)
g)
h)

15. Identify the multiplicity (singlet, doublet, triplet, etc.) for each proton group in the following compounds.
a) CH3CHO
b) CH3CH2Br
c) CH3-O-CH2CH2CH3
d) (CH3)3C-CH3
e) (CH3)2CHCl
f) Br-CH2CH2-CN
g) CH3-Br
16. Sketch the 1H NMR and 13C NMR spectra for each compound.
a) CH3OH
b) chloroethane
c) 1-bromopropane
d) CH3CH2OCH3
e) CH3CH2CH22CO2H
17. Listed here are 1H NMR absorption peaks for three compounds, along with empirical formula and a key IR absorption (if applicable) as well. The following, abbreviations are typically employed: s = singlet; d = doublet; t = triplet; q = quartet; m = multiplet. Please provide structures consistent with these data.





18. A 1H NMR spectrum was taken of an alcohol. The sample was then shaken with D2O. Another spectrum was acquired. What does this tell you about the peak labeled with the blue arrow in the original spectrum? What is this new peak labeled with the red arrow in the second spectrum?


19. Match each of the following compounds with its 1H NMR spectrum.



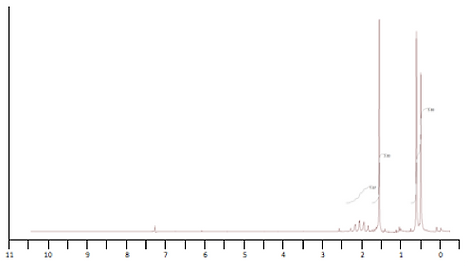

20.
a) Draw the structure of the following ester (formula C5H10O2) given its 1H and 13C NMR spectra.


b) Once you have identified the complete structure of this compound, fill in the lines below to complete the DEPT-45, DEPT-90, and DEPT-135 spectra. Be sure to consider all five carbon peaks.

21. Draw the structure of the following compound with the formula C3H6O given its 13C NMR and DEPT spectra.

22. An ester with the formula C10H12O2 has the following NMR spectra. Draw its structure.


23. A compound with the formula C5H10O has the following 1H and 13C NMR spectra. Draw its structure.


24. A compound with an M+ of 86 in its mass spectrum has the following spectra.