Enolate Problems
Extra Problems
13. What are the products of the following reactions?

14. What are the products when the following reagents undergo aldol condensations following dehydration?

15. What molecule undergoes an aldol condensation to form the following products?

16. Provide the structure of the product of the crossed Aldol condensation between benzaldehyde and acetone.
17. a) Identify the two molecules that react in a crossed aldol reaction to produce the following? b) Identify which of the two molecules would be used in excess in the crossed aldol reaction.

18. What are the products when the following reagents undergo Claisen condensations?

19. Provide the structure of the product of the crossed Claisen condensation between PhCO2CH2CH3 and CH3CH2CO2CH2CH3.
20. Provide the structure of the product of the following Dieckmann condensation.

21. What ester would undergo a Claisen condensation to make the following products?

22. Show how the following compound could be synthesized from a Dieckmann condensation.

23. Show how you would use an aldol reaction to make the following.

24. a) What reagents would you use to make the β-keto ester shown? It is the first part of the acetoacetic acid ester synthesis.

b) Show the ketone that would result from hydrolysis-decarboxylation of the above β-keto esters. (This is the final part of the acetoacetic ester synthesis).
25. Predict the products of the following aldol condensation. Show the products both before and after dehydration.

26. The following crossed aldol reaction is not a reasonable way to synthesize the product shown. Why?

27. a) Identify the two esters that react in a crossed Claisen condensation to produce the following?
b) Identify which of the two molecules would be used in excess in the crossed Claisen reaction.

28. Draw the structures of the products of the following crossed Claisen condensations. Indicate if any pair of compounds would be a poor choice to perform a crossed Claisen condensation.
a) PhCO2CH3 + PhCH2CO2CH3
b) (CH3)3CCO2CH2CH3 + CH3CH2CO2CH2CH3
c) CH3CH2CO2CH3 + CH3CO2CH3
29. Provide the structures of the products of the following reactions.

30. Show how you could use the acetoacetic ester synthesis or the malonic ester synthesis to make the following compounds.
a) 2-hexanone
b) hexanoic acid
c) 4-methyl-2-pentanone
d) 4-methylpentanoic acid
31. Draw the product of the following compounds after they undergo hydrolysis and decarboxylation.

32. Draw the structures of the products of the following reactions.

33. Show the mechanism of the following reaction.

34. Show what starting material(s) you would use to get the following products. Use aldol or Claisen reactions.

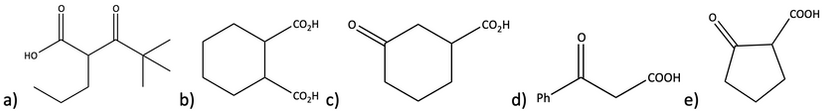
35. Which of the following, when heated, can decarboxylate and lose CO2? If it can decarboxylate, show the product that is formed.
36. Show the products of the following Michael addition reactions.

37. Show how the following compounds can be synthesized by a Michael addition reaction.

