Alkyl Halides &
Free Radicals
Introduction
A good starting point for learning organic chemistry reactions is to investigate organohalides. Organohalides are organic compounds that contain a halogen atom. They can be used to synthesize many different compounds. This is the first step into a wonderful world of making organic compounds. From new materials to life-saving medicines, synthetic organic chemistry is incredibly important to the human condition.
Organohalides can be categorized into several broad categories.

Generic organohalides
Nomenclature
Trivial Names
There are some trivial names for organohalides. The common name typically takes the form of “alkyl halide”.

Some halogenated solvents are very common solvents used in the organic chemistry lab. Methylene chloride (or dichloromethane) is a very common solvent. At one time, methylene chloride was used to decaffeinate coffee. There were concerns that traces of it remaining in the coffee might cause cancer. This fear was largely unfounded as evidenced by animal studies. But, methylene chloride did extract some of the compounds that add to the wonderful taste and flavor of coffee. For these reasons, coffee is now decaffeinated using liquid carbon dioxide, so drink up.
Methylene chloride is still often used in the organic chemistry lab as a solvent. Chloroform is also often used in the organic lab and it was used industrially as a degreaser. It is more toxic than methylene chloride and a stronger carcinogen. It was also used historically as an anesthetic. Today, Halothane, CF3CHClBr is used as a general anesthetic because it is less toxic. Carbon tetrachloride is an excellent spot remover and was used as the primary dry-cleaning solvent for many years. Again, it is toxic and a carcinogen so the less harmful 1,1,1-trichloroethane has replaced it.

Common chlorinated solvents
IUPAC Nomenclature
The IUPAC system used the prefixes fluoro-, chloro-, bromo-, and iodo- followed by the alkane name to name alkyl halides.

Dihalides
If two halides are on the same carbon atom of a molecule, the molecule is a geminal dihalide. If two halides are on neighboring carbon atoms of a molecule, the molecule is a vicinal dihalide.

X = halide
1. Name the following compounds using IUPAC nomenclature.




Preparation of Alkyl Halides
Free radical Halogenation
Industrially, alkyl halides are usually produced through a mechanism called free radical halogenation. In this reaction, a hydrogen atom of an alkane is replaced by a halogen. Free radicals are, of course, involved in the mechanism. There are three steps in this mechanism that you should memorize.
The initiation step of free radical halogenation generates free radicals. In free radical halogenation, this usually means generating Cl· or Br· by shining ultraviolet light on Cl2 or Br2. Once the reaction is initiated, a chain reaction occurs. This chain reaction is a two-step process called propagation. First, the chlorine radical reacts with the alkane. It pulls off a hydrogen atom generating an alkyl radical. This alkyl radical then reacts with Cl2 to make the alkyl halide and another chlorine radical. Look closely at the propagation step. Notice that in each of the two propagation steps, a radical is produced. Radicals are always produced in propagation in order to propagate the chain reaction. For most of the reaction’s life, a radical is most likely to bump into another molecule that is not a radical. The propagation continues. But, at the end of the reaction, the number of molecules that are not radicals gets low. At that point, the radicals in the reaction mixture are more likely to find another radical and combine together.
The termination step is when two radicals combine. A radical is not produced, so the reaction is not propagated, but it is terminated.
Step 1. Initiation (Getting it going by generating radicals.)
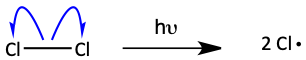
Step 2. Propagation (A chain reaction that keeps making radicals.)

Step 3. Termination (Putting a stop to it all because it combines two radicals and does not make a radical.)

Chlorination of Propane
Sometimes, there is more than one type of hydrogen that can be replaced by the halogen. In propane, there are two secondary hydrogen atoms and six primary hydrogen atoms. If the chlorination of propane is a totally random process, we would expect more primary chloride (1-chloropropane) than secondary chloride (2-chloropropane) in a 6:2 (3:1) ratio. But, when the reaction is performed, more of the secondary chloride is produced! Why is this? When we look at the mechanism for the chlorination, we see that a secondary radical or a primary radical are formed. We’ve learned that secondary radicals are lower in energy and more likely to be made because they are better and more stable than primary radicals (through hyperconjugation).

We can see why more of the secondary radical is produced by looking at the reaction-energy diagram. It is easier to slip over the secondary transition state because the activation energy is lower. It is more difficult to slip over the primary transition state because the activation energy is higher. It is more difficult, but not impossible, to slip over the primary transition state. That is why we get 40% 1-chloropropane. Knowing that if the process was random, we would expect 3:1 primary:secondary but we actually get 60:40 secondary:primary, chemists can do some math to get an understanding of how much more reactive secondary radicals are than primary radicals in a free radical chlorination reaction. Chemists have done many such reactions and have found that tertiary radicals form 5 times faster than primary radicals and secondary radicals form 3.8 times faster than primary radicals.

Reaction coordinate diagram for the chlorination of propane
Bromination of Propane
In the bromination of propane, we have similar considerations to think about. Once again, there are six primary hydrogen atoms and two secondary hydrogen atoms on propane. If the process was completely random, we would expect a ratio of 6:2 (3:1) 1-bromopropane:2-bromopropane. When the experiment is performed, we find the following results.

We get 97% of 2-bromopropane! This is a much higher percentage than in the chlorination reaction. We say that this reaction is more selective. The secondary radical is highly favored over the primary radical in this bromination reaction. Why is this? The reaction-energy diagram helps explain it. Unlike the chlorination reaction which is exothermic, the bromination reaction is endothermic. Because of this, there ends up being a bigger gap between the energies of the two transition states than there was in the previous chlorination reaction.
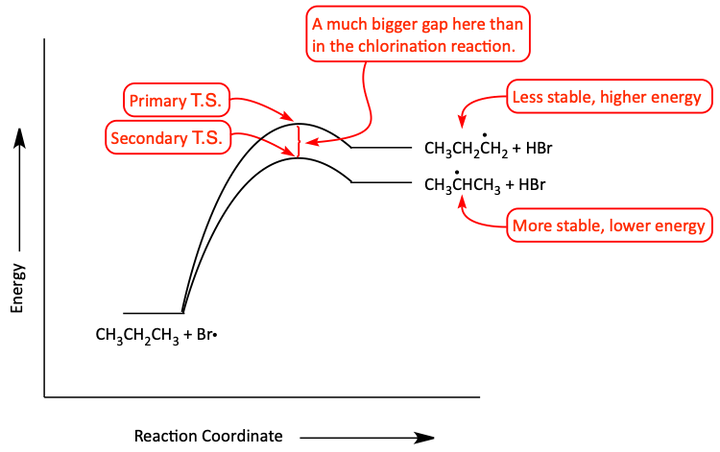
Reaction coordinate diagram for the bromination of propane
After chemists have done the experiments and math on bromination reactions, they found that tertiary radicals are 1600 times more reactive than primary radicals. Secondary radicals are 82 times more reactive than primary radicals. So, in bromination free radical reactions, the most stable free radical sites are where the bromination usually occurs.
The reason that there is a bigger difference in the energies of the transition states in an endothermic reaction over an exothermic reaction is because of The Hammond Postulate. The Hammond Postulate states that the transition state’s structure is closest to the species of similar energy. For example, in the exothermic chlorination reaction, both the primary and secondary transition states are similar in structure to the starting propane material. In the endothermic bromination reaction, the transition states are more similar to the free radical products. This causes the difference between the two energies of the transition states to be larger.


Allylic and Benzylic Radicals
Let’s look at free radical reactions when there is a pi-bond present. When cyclohexene reacts with a bromine radical, the following product predominates.

Let’s analyze the hydrogen atoms that we could have replaced on cyclohexene. All of the hydrogen atoms on cyclohexene are secondary, yet their reactivity is very different. The hydrogen atoms on the double bond are called vinyl or vinylic hydrogen atoms. Hydrogen atoms on a carbon that neighbors the double bond are called allyl or allylic hydrogen atoms. This carbon is an allylic carbon atom. The other hydrogen atoms are regular alkyl hydrogen atoms. Allylic hydrogen atoms are the most reactive. Why is this since they are all secondary hydrogens? They are all secondary because each of the hydrogen atoms is on a carbon atom that is attached to two carbon atoms.

Vinyl vs. allyl vs. alkyl protons
If we look at the radical that is formed when the allylic hydrogen atom is removed, we find that resonance forms can be drawn for it. This greatly stabilizes the free radical.

Free radical bromination at an allylic position
The 2-bromocyclohexenes that were formed above were drawn flat. In fact, they are not flat. A racemic mixture of two enantiomers is formed.
2. Draw a star on the stereogenic center of 2-bromocyclohexene. Then, draw the two enantiomers of 2-bromocyclohexene. Hint: you may want to draw in a missing hydrogen atom.

If a vinylic or alkyl hydrogen are removed, resonance forms cannot be drawn. Vinylic radicals are especially unstable. In a vinyl radical (much like in a vinyl cation), the electron deficient partially positive location is in an sp2 hybrid orbital and held closer to the positive carbon nucleus than it is in an sp3 hybrid orbital. This is a bad situation.
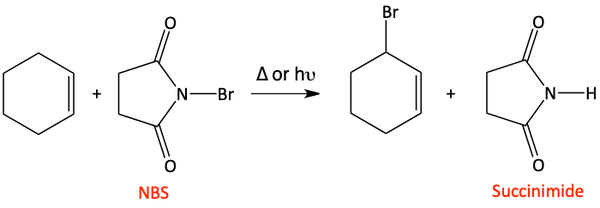
When we generate bromine radicals in the presence of a double bond, we need to be careful. If we did an initiation reaction with Br2 and ultraviolet light like we have already learned, we would run into problems. The problem is that alkenes react with Br2. We’ll learn more about this in the alkene chapter, but a dibromination of the double bond can happen.


How do we avoid this problem? We need to make sure we don’t have too much Br2 present in our reaction mixture. The best way to do this is to perform this reaction with NBS (N-bromosuccinimide) and not Br2. NBS generates Br2 in small amounts so the dibromination of the double bond does not happen.
Now that you know about allylic bromination, predict the product of the following reaction.
3. Predict the product of the following reaction.

We call the carbon that neighbors a benzene ring a benzylic carbon atom. The hydrogen atoms on that carbon are benzylic hydrogen atoms.

We can now include these resonance-stabilized positions in a rank of the stability of free radicals.

Stability of free radicals
Always consider the various resonance forms when a free radical reaction occurs in a compound with a pi-bond. Sometimes, if the molecule is symmetrical, the products we get are the same, so it does not matter. We saw this in the bromination of cyclohexene above and in the following bromination of propene.

So, if we draw the overall reaction, we only need to draw one product.
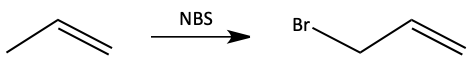
But, when the alkene is less symmetrical, it can lead to a mixture of products.

Since the products are different, we need to include both products when we draw out the overall reaction.

4. Identify the most reactive hydrogen atom(s) in the following molecules if they undergo free radical reactions.



5. For the three molecules in the previous problem, draw the monobrominated product(s) of each if they undergo a free radical bromination. Monobrominated means only one bromine atom is on the molecule.
Answers
1.

2.

3.

4.

5.



