Organic Reaction Basics
Types of Reactions
Organic reactions can be placed into several different categories.
1. Substitutions. Much like how a substitute teacher replaces the regular teacher, a substitution reaction is one where one atom or group is replaced, or substituted, with another atom or group. One group replaces another group. In this case, hydroxide (OH-) replaces chloride (Cl-).
2. Additions. In an addition, two atoms or groups are added to a molecule’s π bond. In effect, two molecules are added together. Two molecules become one molecule.

3. Eliminations. When something is eliminated, it is expelled or removed. In an elimination reaction, two atoms or groups are expelled from a molecule to generate pi bond and make two molecules. Elimination reactions are the opposite of addition reactions. Notice in this example, both H and Cl are removed to form a pi bond.

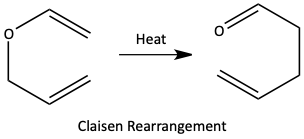
4. Rearrangements. In a rearrangement, a molecule reorganizes its bonds and atoms to make an isomer of itself.
5. Free radical. In a free radical reaction, the reactive species is one that contains a single, unpaired electron (a free radical). Ultraviolet light is often used to generate this free radical.

6. Oxidations/Reductions. In organic oxidations or organic reductions, an organic species either gains oxygen or loses hydrogen. If a molecule makes more carbon-oxygen bonds and/or loses carbon-hydrogen bonds, we say that the organic species is oxidized. If a molecule gains hydrogen bonds, we say it is reduced.

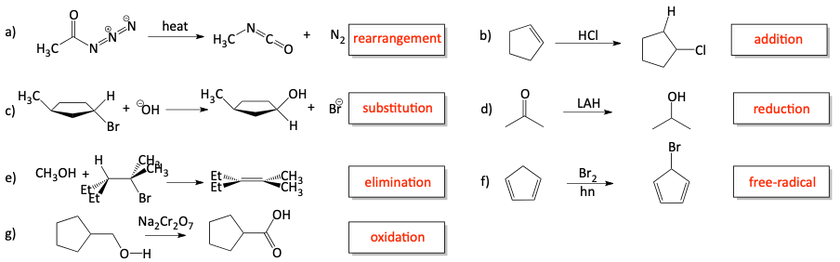
1. Identify each of the following reactions as a substitution, addition, elimination, rearrangement, free radical, oxidation, or reduction.
a)

b)

c)

d)

e)

f)

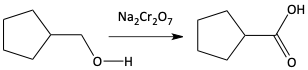
g)
Answers
1.