In the lab
Acids and bases in the lab
In the lab, an unknown compound’s acidity or basicity can help us begin to identify what type of compound we have. To explain how this works, let’s first take a look at how four different compounds react with aqueous acid or base. Of course, aqueous acid or base means the acid or base is dissolved in water. We will use a strong acid like 10% aqueous hydrochloric acid (10% HCl), a strong base like 10% aqueous sodium hydroxide (10% NaOH), and a weaker base like 10% aqueous sodium bicarbonate or baking soda (10% NaHCO3). In each case, when an organic compound reacts with the acid or base, it forms a salt (a positive cation with a negative anion). Salts are soluble in water. This is key for us.
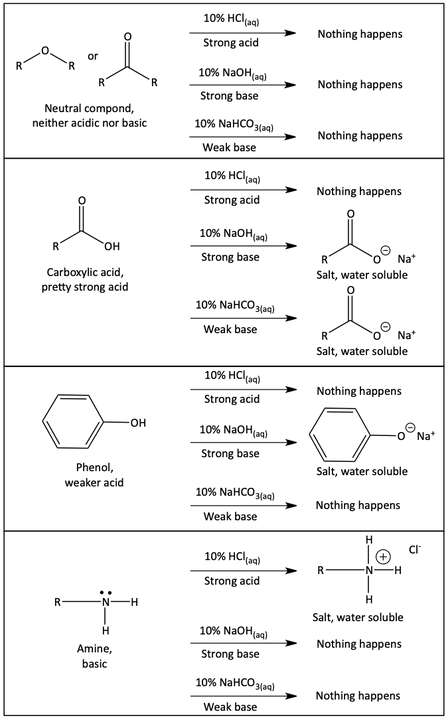
Notice how a neutral compound will not react with any of the acids or bases. It will not dissolve in any of the aqueous acids or bases. A fairly strong acid, like a carboxylic acid, will not dissolve in aqueous acid (HCl), but can react with a strong base (NaOH) or will even react with a weak base (NaHCO3) forming a salt that will dissolve in the aqueous solution. A weaker acid, like phenol, will not react with aqueous acid (HCl) or a weak base (NaHCO3). Therefore, it will not dissolve in those aqueous solutions. But, a weak acid like phenol can react with a strong aqueous base (NaOH) to form a salt and dissolve. Finally, a basic compound like an amine will react with aqueous acid (HCl) to form a salt and dissolve, but will not react the bases. Study the following chart until it makes sense to you.
Extractions, using separatory funnels, is a separation technique in the organic chemistry lab that uses this idea of different compounds forming water-soluble salts with various acids or bases. This is a very common organic lab technique. These are crucial ideas to understand.