Sigma and Pi Bonds
When two atoms come together, the electron orbitals on the atoms can overlap. This constructive overlap of electron density between two atoms makes bonding molecular orbitals, also called bonds. There are two main types of bonds we will deal with in organic chemistry, sigma and pi bonds. A sigma bond is directly between two atomic nuclei. Or, a more technical way to describe it is the electron density lies on the internuclear axis. A pi bond has electron density above and below the internuclear axis.

Sigma and pi-bonds
A mnemonic to help you learn these at first is to think of the first, single bond between two atoms as a snake, the sigma snake. This can help you think of a sigma bond. The second bond is split into two regions, above and below the internuclear axis. A pi bond may remind you of a pie crust.

Sigma and pi-bond mnemonics
We’ve learned that hybridization is a mixing of atomic orbitals on one atom. We need to look at how two or more atoms come together to make bonds and form molecules. We mix hybrid and/or atomic orbitals to make these molecular orbitals. These bonds that we form between atoms are called molecular bonds.
Atomic orbitals are s and p orbitals on individual atoms.
Hybrid orbitals are sp, sp2, s p3 orbitals that are mixed from the s and p atomic orbitals on the same atom.
Molecular orbitals are a mixing of atomic and hybrid orbitals between different atoms to make bonds between those atoms. This is how we make molecules.
Alkane bonding
Let’s look at how some simple molecules are put together. Let’s begin with propane.
Let’s redraw it in 3D. We see that each of the carbon atoms has four bonds and is sp3 hybridized.


First, let’s draw the central carbon atom with its four sp3 hybrid orbitals around it. We draw it with the bottom two sp3 hybrid orbitals pointing towards the bottom right and bottom left so they are pointing towards where the other carbon atoms will be drawn. Notice, when we draw the hybrid orbitals, we typically leave off the small back lobes on each one. This makes it easier to draw and makes it less complicated.
Now, let’s draw the other, outside, carbon atoms with four sp3 hybrid orbitals around each one.
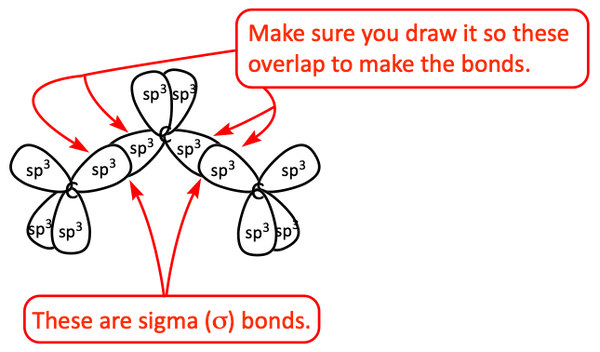
A sigma bond is made by two hybrid orbitals overlapping directly between the nuclei of two atoms.
Finally, we draw in the hydrogen atoms. A hydrogen atom cannot make hybrid orbitals because it is just one 1s orbital. They are simple to draw. We draw the spherical 1s orbitals for each hydrogen atom. Notice, they overlap with the hybrid orbitals on the carbon atoms.

Orbital representation for propane
Alkene bonding
Let’s look at something with a double bond in it, ethene.

We identify the hybridization of the atoms.
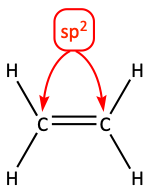
Then, we draw in the sp2 hybrid orbitals around the carbon atoms making sure we arrange them so they overlap between the carbon atoms. If they do not overlap, they do not make a bond.

Pi bonds
But, whenever we have sp2 hybridization, there is one atomic p-orbital on each carbon that remains unhybridized. If we draw those in, we see that they can overlap above and below the carbon-carbon internuclear axis. They make a pi bond (π-bond).

Notice, the σ-bond is the one that lies directly between the carbon nuclei. Notice the π-bond is above and below the internuclear axis (between the carbon nuclei). Half of the π-bond is above the plane and the other half is below the plane. These two parts together make one π-bond. So, there is one σ-bond and one π-bond between the carbon atoms. This is how we get a double bond. A double bond consists of one σ-bond and one π-bond.

Orbital representation for ethene
Alkyne bonding
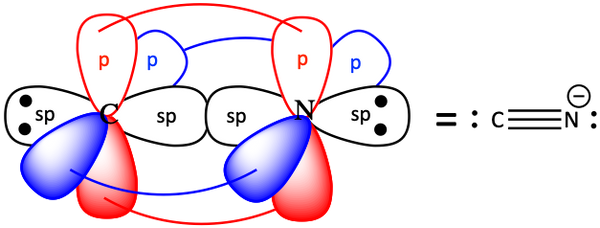
For acetylene, H-C≡C-H, the carbons are sp hybridized. There are two unhybridized p-orbitals on each carbon that can overlap to make two π-bonds.

Orbital representation for ethyne
Important point
The first bond (the single bond) is a σ-bond. The second and third bonds of a multiple bond (double or triple bond) are π-bonds.
2. Identify the number of sigma bonds and pi bonds at the indicated bonding sites.

3. Draw an orbital representation for the cyanide ion, :C≡N:-.
Answers
2.

3.