top of page
Alcohol Synthesis Summary
Reaction Summary
Thiol synthesis via SN2 with sodium hydrosulfide
a)

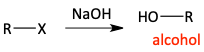
Alcohol synthesis via SN2 with hydroxide
b)
Acid hydrolysis of an alkene
c)

Markovnikov alcohol
Markovnikov Oxymercuration/Demercuration of an alkene in water
d)

Markovnikov alcohol
Anti-Markovnikov hydroboration of an alkene
e)
Anti-Markovnikov alcohol

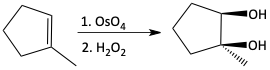
Syn-dihydroxylation of an alkene using osmium tetroxide
f)
cis glycol
Syn-dihydroxylation of an alkene using potassium permanganate
g)

cis glycol
Epoxidation of an alkene followed by acid hydrolysis
h)

trans glycol
Acetylide attack of an epoxide
i)

Acetylide attack of a carbonyl
j)

Synthesis of a Grignard reagent
k)

Synthesis of organolithium reagent
l)

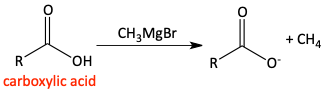
Grignard reagent attacks of carbonyls
m)

n)

o)

p)

q)
Grignard attack of epoxides
r)

Synthesis of a Gilman reagent
s)
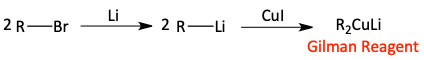
Gilman reagent with alkyl halide
t)
Reductions of carbonyls
u)
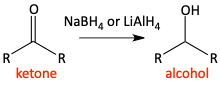
v)

w)

x)

y)

z)

bottom of page
