Hydrogenation
Syn-addition: Giving it the horns
In some alkene addition reactions, the product is the result of syn-addition. This means the two groups added across the pi-bond both end up on the same side. I call such reactions “Giving it the horns” reactions. I do so because I use a mental image of a bull with its horns goring the alkene. Its two horns go into the alkene and break off leaving the horns sticking out on the same side of the alkene.


Syn-addition. Giving it the horns.
Hydrogenation - Giving it the horns.
Of an alkene
Hydrogenation is the reaction of an alkene with hydrogen gas in the presence of a metal catalyst. The metal catalyst used is often platinum (Pt), palladium (Pd), or Nickel (Ni). The hydrogen adds across the pi-bond turning the alkene into an alkane. The two hydrogen atoms add to the same side of the alkene in a syn-addition fashion.

For the mechanism of hydrogenation, the hydrogen first adds to the metal catalyst. The alkene then adsorbs onto the metal catalyst. One carbon atom of the alkene inserts into the metal-hydrogen bond and then the other. Since both hydrogen atoms come from the same metal catalyst surface, both hydrogen atoms end up on the same side of the alkene. This is syn-addition. The hydrogen atoms on the metal catalyst are “Giving it the horns.”

Of an alkyne
Hydrogenation to an alkane
Alkynes have two pi-bonds compared to the one in an alkene. Therefore, the hydrogenation of an alkyne can occur twice to form a saturated alkane. Usually, good metal catalysts like Pt, Pd, or Ni are used. The hydrogen atoms on the metal catalyst are “Giving it the horns.”



Hydrogenation to an alkane
But, what if you don’t want to hydrogenate an alkyne all the way down to an alkane, but you want to stop halfway at the alkene? Is there a way to only hydrogenate halfway? There is. The key is to use a less effective, partially deactivated catalyst, a poisoned catalyst. Lindlar catalyst is a poisoned catalyst. It is palladium (Pd) coated with barium sulfate powder (BaSO4) in the presence of quinoline. When the hydrogenation of an alkyne takes place in the presence of Lindlar catalyst, an alkene with syn-stereochemistry is formed. It is syn-addition because both hydrogen atoms again come from the same Pd catalyst surface. The hydrogen atoms on the metal catalyst are “Giving it the horns.” If an internal alkyne is hydrogenated in this way, it leads to cis-alkenes.

A newer reagent, nickel boride, Ni2B, is becoming more popular to make cis-alkenes from alkynes. It is easier to make and works a little better than Lindlar catalyst.
Hydrogenation to trans-alkene
So, this begs the question, can we start with an alkyne and end up with a trans-alkene? We can. But, we need to change the mechanism of how the hydrogenation occurs. We can no longer use a poisoned metal catalyst because, if we do, both hydrogen atoms will add by “Giving it the horns” making cis-alkenes. One hydrogen atom needs to add at a time to be able to get trans-alkenes.
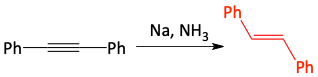
Sodium metal in liquid ammonia can turn an alkyne into a trans-alkene.

Let’s walk through the mechanism to see why this works.
1. Overall, this reaction is a reduction. Reductions need electrons. It would be nice if we could simply buy a bottle of electrons. Since we cannot, we need to make some. Reacting sodium and ammonia is a good way to make a beaker full of electrons.
For this reaction, sodium metal is dissolved in liquid ammonia. Ammonia has a boiling point of -33°C. It is a gas at room temperature. In order to turn the gaseous ammonia into a liquid solvent, it is cooled in a special chamber using dry ice. Once the ammonia is a liquid, pieces of solid sodium metal are added. Once enough sodium is added, it turns a beautiful blue color. This blue color means we have solvated electrons.

How does this work? Sodium metal is an alkali metal. It wants to give up one electron to become Na+. This is the source of the electrons. Ammonia has the perfect molecular shape to hold these electrons. Ammonia’s trigonal pyramidal shape forms a pocket with the positively charged hydrogen atoms that can hold a negatively charged electron. These freed electrons can be thought of as “electrons in a bottle”.

2. The free electron attacks a carbon atom of the alkyne. This would put too many electrons on the carbon atom, so the pi-bond leaves to form a lone pair of electrons making an anionic intermediate. The species formed is a radical anion because it has both a single electron (the radical) and a lone pair of electrons (the anion). Since the free electron attacking and the lone pair of electrons leaving are both negatively charged, they get as far away from each other as possible, in a trans configuration.

3. In this mechanism, whenever an anionic lone pair of electrons is formed, the lone pair goes out and grabs a proton. In this mechanism, they get the proton from ammonia and form a vinyl radical. This is a radical (a single electron) on a double bond.

4. Once the neutral vinyl radical is formed, another electron from the solvated electrons attacks. It joins the single electron to make a lone pair anion. Since this anion is directly on a double bond, it is called a vinyl anion.

5. Again, whenever the lone pair anion is formed, it grabs a proton. The ammonia once again acts as a proton source. This results in the desired trans-alkene.

Instead of using sodium and ammonia, another reagent that can form a trans-alkene from an alkyne is lithium aluminum hydride, LiAlH4 or LAH.
8. Draw the product of the following reactions.
a)

b)

c)

d)

e)
f)
g)
Answers
8.
a)

b)
c)

d)

e)
f)

g)

