Alkene & Alkyne Reaction Problems
Extra Problems
14. Draw the products of the following reactions.
a) 1-methylcyclopentene + HBr
b) 1-methylcyclohexene + HBr + PhOOPh
c) 1-butene + HCl
d) 2-methylbutene + HI
e) 1-ethylcyclopentene + HBr + BuOOBu
f) propene + HBr
g) propyne + 2 HBr
h) butyne + HBr + PhOOPh
i) pentyne + 2 HI
15. Identify the appropriate reagents needed to perform the following reactions.

16. Show the mechanism for the following reactions.

17. When 1-pentyne reacts with HgSO4 in sulfuric acid, a Markovnikov enol is first formed.
a) Draw the enol.
b) Then, draw the mechanism for the acid catalyzed keto-enol tautomerization to turn the enol into a ketone. Remember to protonate and go to town.
18. The alkene below was reacted with BH3 followed by H2O2/OH-. Give the product of this reaction.

19. What reagent would perform the following reactions?
a) 3,3-dimethylbutene to 1-bromo-3,3-dimethylbutane, (CH3)3CCH2CH2Br
b) 1-butene to 1-butanol
c) 3,3-dimethylbutene to 2-bromo-3,3-dimethylbutane, (CH3)3CCH(Br)CH3
d) 1-pentene to 2-pentanol
e) 3,3-dimethylbutene to 3,3-dimethylbutanol, (CH3)3CCH2CH2OH
f) 1-pentene to 1-pentanol
g) 3,3-dimethylbutene to 3,3-dimethylbutan-2-ol, (CH3)3CCH(OH)CH3
h) For part g), it is a bad idea to use dilute aqueous H2SO4 to do that reaction. Why?
i) 3,3-dimethylbutene to 3,3-dimethyl-2-methoxybutane, (CH3)3CCH(OCH3)CH3
j) 1-butyne to butyraldehyde, CH3CH2CH2CHO
k) 1-butyne to 2-butanone, CH3COCH2CH3
20. Draw the products of the following reactions.
a) 1-methylcyclopentene + Br2 in CHCl3
b) 1-methylcyclopentene + Cl2 in water
c) 2-methylpropene + Br2 in CH2Cl2
d) 2-methylpropene + Br2 in water
e) 1-butyne + 2 Br2
f) 3-hexyne + 2 Cl2
21. What reagent would perform the following reactions?
a) 3,3-dimethylbutene to 1,2-dibromo-3,3-dimethylbutane
b) 2-octyne to 2,2,3,3-tetrachlorooctane
c) propyne to 1,1,2,2-tetrabromopropane
d) 1-methylcyclohexene to 2-bromo-1-methylcyclohexanol
e) 3,3-dimethylbutene to 1-chloro-3,3-dimethylbutan-2-ol, (CH3)3CCH(OH)CH2Cl
22. Draw the products of the following reactions.
a) 1-methylcyclopentene + dilute aqueous H2SO4
b) 1-methylcyclopentene + aqueous Hg(OAc)2 followed by NaBH4
c) 1-methylcyclopentene + Hg(OAc)2 in CH3CH2OH followed by NaBH4
d) 1-methylcyclopentene + BH3.THF followed by H2O2 and NaOH
e) 2-methylpropene + dilute aqueous H2SO4
f) 2-methylpropene + aqueous Hg(OAc)2 followed by NaBH4
g) 2-methylpropene + Hg(OAc)2 in CH3CH2OH followed by NaBH4
h) 2-methylpropene + BH3.THF followed by H2O2 and NaOH
i) 1-pentyne + HgSO4 in sulfuric acid
j) 1-pentyne + disiamylborane followed by H2O2 and NaOH
23. Draw the products of the following carbene reactions.

24. Draw the alkene and other appropriate, carbene-generating reagents, needed to perform make the following compounds.

25. Regarding the following reaction:

a) List the reagent(s) that could accomplish this reaction.
b) What is the reactive species, the intermediate, in this reaction called?
26. Draw the mechanism for the following reaction.

27. Draw the products of the following reactions.
a) 1,3-cyclohexadiene + H2/Pt
b) trans-2-pentene + H2/Ni
c) 1,4-pentadiene + excess H2/Pt
d) 2-pentyne + H2 Lindlar catalyst
e) 2-pentyne + Na in liquid NH3
f) 2-pentyne + excess H2/Pt
g) 3-hexyne + H2 + Pd/BaSO4 + quinoline
28. The hydrogenation of the alkene below could theoretically produce two possible diastereomers (A and B). Only one, A, is formed. Explain why.

29. Draw the products of the following epoxidation reactions.
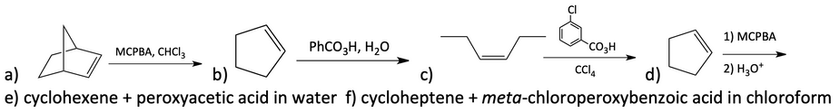
30. Identify the alkene and appropriate reagents to make the following compounds.

31. What are the products of the following alkene oxidations?

32. Identify the alkene or alkyne and appropriate oxidation reagents to make the following compounds.

33. What would be the product of reacting the 2-methyl-2-pentene with:
a) OsO4 followed by H2O2?
b) Ozone followed by dimethylsulfide (Me2S)?
c) MCPBA in CHCl3
d) peroxyacetic acid in H2O
e) KMnO4, neutral, dil.
f) KMnO4, warm, basic
g) H2, Pt
h) Simmons-Smith reagent
i) chloroform and sodium hydroxide
j) Br2 in CCl4
k) Br2 in water
l) HBr
m) HBr and benzoyl peroxide
n) BH3.THF followed by H2O2/OH-
o) Hg(OAc)2 in water
p) Hg(OAc)2 in CH3OH
34. Orlon yarn is fabric that can be knitted into sweaters. Orlon is the trade name for polyacrylonitrile. It is synthesized through a free-radical polymerization of acrylonitrile. Draw the mechanism for this polymerization, and draw the structure of the polymer.

35. Give the alkene monomer that can be used to make the following polymers.
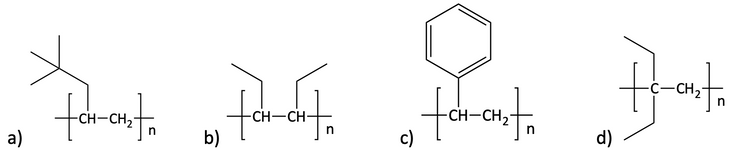
36. Draw the polymer created from the addition reactions of each of the following alkene monomers.

37. Give the products formed in the following olefin metathesis reactions.

38. A laboratory wants to make a shorter version of the following fatty acid ester. They react it with a smaller alkene in the presence of Grubbs catalyst.
a) Draw the structure of the shorter ester they made.

b) Olefin metathesis can also be used to extend (or make longer) a fatty acid ester. The same fatty acid ester is reacted with a longer alkene in the presence of Grubbs catalyst. Draw this longer fatty acid ester.

39. An unknown cyclic alkene, A, underwent a series of reactions shown below. Identify compounds A-I.

40. Provide the reagents necessary to complete the following transformations. (More than one step will be required for each.)
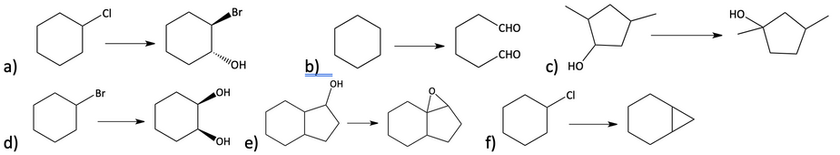
41. Show how you would synthesize the following alkynes starting with acetylene.
a) 1-butyne
b) 2-pentyne
c) 3-hexyne
d) 2-methyl-3-heptyne
42. What reagents would perform the following reactions?
a) 2-pentyne to cis-2-pentene
b) 3-hexyne to trans-3-hexene
c) 1-butyne to butyraldehyde (CH3CH2CH2CHO)
d) 1-pentyne to 2-pentanone (CH3COCH2CH2CH3)
e) 2-hexyne to hexane
f) 1-butyne to 2,2-dichlorobutane
g) 1-propyne to 2-hexyne
h) 3-hexyne to cis-3-hexene
i) 2-pentyne to 2,3-pentandione (CH3COCOCH2CH3)
j) 1-pentyne to a mixture of cis- and trans-1-bromopentene
k) 2-butyne to CH3CO2H (two ways)
l) 2-pentyne to 2,2,3,3-tetrabromopentane
m) 2-butyne to trans-2-butene
n) 3-octyne to octane
43. Show the products of the following alkynes following reactions with 1) HgSO4 (mercuric sulfate) and sulfuric acid, 2) disiamylborane followed by hydrogen peroxide and sodium hydroxide, 3) H2 and platinum, 4) H2 and Lindlar catalyst 5) Na and NH3, 6) dilute, neutral KMnO4, and 7) warm, concentrated, basic KMnO4.
a) 1-butyne
b) 2-butyne
c) 1-hexyne
d) 2-hexyne
e) 3-hexyne
44. What sequence of three reactions can you use to turn trans-3-hexene into cis-3-hexene? Making an alkyne is helpful.
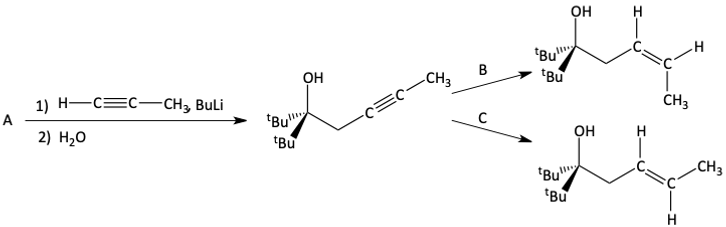
45. Draw the product of the following reaction.

46. What is A, B, and C in the following reactions?
47. Show how you would synthesize the following compounds starting with acetylene and any necessary additional reagents.

48. Show what alkyne, when it undergoes ozonolysis, will make the following carboxylic acids.
a) alkyne that makes CH3CH2COOH + CH3COOH
b) alkyne that makes HOOCCH2CH2CH2COOH + 2 CH3COOH
49.
a) Show how you would convert 2-bromopropane to 1,2-dibromopropane in two steps.
b) Show you would convert 1-methylcyclohexanol to 1-bromo-2-methylcyclohexane in two steps.
50. Draw the products for each of the following reactions.

51. Draw in the reagents/reactants for each of the following reactions.
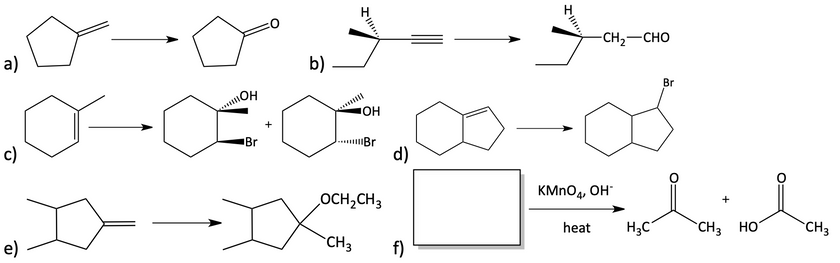
52. Starting from molecules containing only five carbon atoms or less, draw the chemical reactions needed to show how you would synthesize the following compound.

53. A student was given an unknown compound (A) for an organic chemistry lab. The student was told the formula for unknown A was C6H11Cl.
a) The student determined that unknown A contained how many degrees of unsaturation?
The student then took unknown A and reacted it with a bromine solution. Nothing happened. Then, the student attempted to react A with a KMnO4 solution. Nothing happened.
b) Think about what you’ve learned in this chapter that reacts with bromine and/or KMnO4. What does this lack of reactivity tell the student about unknown A? Is the degree of unsaturation for unknown A from a pi bond or something else? If not a pi bond, then what?
Upon treatment of unknown A with tBuOK, a product, B, with the formula C6H10 was formed.
c) How many degrees of unsaturation does compound B have?
Compound B reacts with bromine (turning the brown solution to colorless) and reacts with cold and dilute KMnO4 (turning the purple solution to brown).
d) What do these reactions tell us about compound B?
Compound B reacts with H2 and Pt to form a compound, C, with the formula C6H12. Compound C shows only one peak in its 13C NMR spectrum.
e) How many types of carbon atoms are in compound C?
f) How many degrees of unsaturation does C have?
The student reacts B with a more concentrated solution of KMnO4 and warms it up to make compound D, C6H10O4.
g) Propose structures for A, B, C, and D.
54. An unknown compound, E, decolorizes a solution of bromine in CCl4.
a) What does this tell us about compound E?
Unknown E undergoes hydrogenation (H2, Pt) to make:
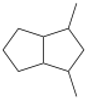
Additionally, E undergoes ozonolysis to make 1,2-cyclopentanedione,

and 2,4-pentanedione,

b) What is the structure of E?
55. a) What is the reagent, A, in the following reaction?

b) What two reagents would you use (one is a terminal alkyne) to make Li-C≡C-TMS.
56. An unknown alkyne, A, underwent a series of reactions shown below. Identify compounds A-G.

