Acetylides
Acetylide formation
Terminal alkynes are slightly acidic. This is because the proton is on an sp hybridized carbon atom. Once deprotonated, this carbon atom will hold its negative lone pair of electrons in an sp hybrid orbital that is 50% s-character. These negative electrons will be held close to the carbon atom’s positive nucleus. You can review this in the acid/base discussion here. The resulting anionic alkyne is called an acetylide.

Terminal alkynes are only slightly acidic, though. A fairly strong base is required to deprotonate it. Negative oxygen bases like hydroxide, HO-, or alkoxide, RO-, simply do not work. Stronger bases are required. Reactive bases with negative nitrogen atoms or negative carbon atoms are needed to deprotonate terminal alkynes.

Sometimes, the acetylide ion will be drawn with its counterion. It may be drawn in the following ways.


Reactions of acetylides
Acetylides are good nucleophiles and good bases. For example, they can act as the nucleophile in an SN2 reaction or a base in an E2 reaction.
SN2 Reaction of Acetylides
The negative acetylide is a very good nucleophile. It can attack the backside of a substrate with a good leaving group. Like in any SN2 reaction, this only works if the backside attack is not sterically hindered. It is best if R’ is methyl, primary, or possibly secondary, but not tertiary.

The SN2 reactions of acetylides can be an effective way to make whatever alkynes or alkenes you might desire. The simplest alkyne, acetylene, can be deprotonated twice. The acetylides formed can undergo SN2 reactions with various alkyl halides to make disubstituted alkynes. The disubstituted alkyne can be reduced to either cis or trans alkenes.

E2 Reaction of acetylides
If the backside attack is hindered, an E2 reaction can occur instead. In this case, the acetylide acts as a base and deprotonates.

Acetylide Attacking a Carbonyl
Acetylides can attack carbonyl compounds. We’ll learn more about this in a future chapter, but let’s look at the attack of a ketone for now. The electronegative oxygen atom in a carbonyl attracts negative electron density towards itself making the carbon atom of a carbonyl partially positive.

The nucleophilic, negative acetylide can attack the positively charged carbon atom of a carbonyl. Forming this bond would make five bonds on the carbon atom, so one bond must leave. The pi-bond of the carbonyl can go up onto the oxygen atom, giving it a negative charge. The compound remains this oxide until the reaction is quenched with acid and water, giving it a proton turning it into a neutral alcohol.

Acetylide Attacking an Epoxide
The nucleophilic attack of an acetylide with an epoxide is a triangle reaction. Analyzing the reaction, we see that the oxygen atom of the epoxide is neutral. The acetylide, with its negative charge, is an aggressive nucleophile. This aggressiveness makes it want to attack the less crowded carbon atom of the epoxide. In our triangle reaction analogy, the victim is holding up a sign with an 0 on it representing he is carrying 0 money. Notice the oxygen atom is neutral and not positively charged. This thief is aggressive. Even though the victim has no money, the thief punches the victim in the side. The thief punches the victim in the side that is not protected with bags. It won’t punch in the side with the bags because it is more difficult to do so. There is also no money there to steal.

Once the acetylide attacks the epoxide, it would make five bonds on the carbon atom. Therefore, a bond must leave. The one that leaves is the sigma bond between the attacked carbon atom and the oxygen atom forming a negative oxygen oxide. The compound remains this negative oxide until the reaction is quenched with water and acid forming an alcohol.
Heavy metal acetylides
Terminal alkynes react with silver or copper monovalent cations to form heavy metal acetylides. These silver and copper acetylides are more covalently bonded than what we’ve seen with sodium or lithium acetylides. This makes them less basic and less nucleophilic. Silver and copper acetylides also precipitate out of water. Heavy metal acetylides should be handled with caution. They can explode when they are dry. So, they need to be kept wet.
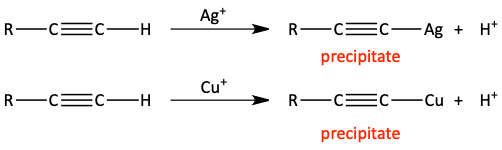
The separation of terminal alkynes from internal alkynes is one use of heavy metal acetylides. Cu+ can be added to a mixture of both terminal and internal alkynes. Only the terminal alkyne will react with the Cu+ forming a copper acetylide precipitate. This precipitate could be filtered out of the solution. This pulls the terminal alkyne out of the solution and leaves the internal alkyne behind.

While the copper acetylide is still wet (remember dry copper acetylide is explosive), acid is added to reform the terminal alkyne.

13. Draw the products of the following reaction.
a)

b)

c)

d)
e)

f)

Answers
13.
a)

b)

c)

d)
e)

f)

