Polymers
Polymers
Polymers (poly from Greek “many”, mer from Greek, méros “part”) are molecules that are long chains made of repeating units. The molecules that are the repeating units are called monomers. Many of our synthetic materials are polymers. Plastic materials are polymers. Alkenes can be monomers.


Polymers are usually represented with a simplified form. The value for “n” is usually some very large number. It represents the number of repeating monomers in the polymer.

Various Polymers
Alkenes undergo addition polymerization using three different mechanisms. These mechanisms are described by their reactive intermediates.
Anionic Polymerization
If an anion is present, like hydroxide, anionic polymerization of the alkene can occur. For an anionic polymerization to occur, the alkene must contain anion-stabilizing groups, also called electron-withdrawing groups. Carbonyl, nitro, and cyano groups are capable of stabilizing carbanions by resonance. Probably the most familiar anionic polymerization is using the monomer methyl α-cyanoacrylate. This compound can readily be purchased in a tube. Once the monomer touches something, like your fingers, a small amount of base that is usually present can attack the alkene and polymerize. Methyl α-cyanoacrylate is commonly called Super Glue. In the anionic mechanism for Super Glue, the anionic hydroxide attacks the alkene with the pi-electrons becoming a lone pair of electrons on a carbanion. This negative pair of electrons can be stabilized through resonance on both the carbonyl and the cyano (nitrile) group. Having two resonance stabilizing groups makes this polymerization occur rapidly.
First step

Subsequent steps


Cationic Polymerization
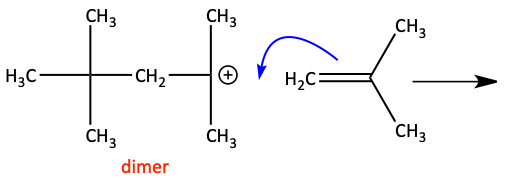
In order to help stabilize the carbocation intermediate, cationic polymerizations usually occur on alkenes with electron donating groups attached. A good electrophile is the initiator of this polymerization. A good Brønsted-Lowry acid (H+) or a good Lewis acid (AlCl3, BF3) works well.
For the mechanism, the negative electrons of the alkene’s pi bond attack the acid to form a carbocation. In the example, the carbocation is tertiary and very stable. Another alkene monomer attacks this electrophilic carbocation to form yet another. This process continues over and over.





Free radical Polymerization
The third polymerization mechanism is a free radical polymerization. An initiation step is the first step of the free radical mechanism. Here, a peroxide is heated to form two oxygen free radicals.

For the free radical mechanism to be successful, an alkene that can stabilize the free radical is needed. In this case, the radical ends up adjacent to a phenyl group making a benzylic free radical. This is quite stable because it can be stabilized by resonance. The reaction keeps repeating until the polymer is formed.







11. Draw the polymers that are made from the following alkene monomers?
a)
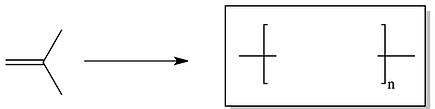
b)

c)

Answers
11.
a)

b)

c)

