Alkenes and Alkynes
Introduction
Alkenes are compounds that contain a carbon-carbon double bond. Years ago, it was common to call alkenes olefins. This term is still heard at times today. Alkenes are found in many natural and biological compounds. Alkenes contain one sigma bond and one pi bond and are sp2 hybridized. The pi bond is more reactive than sigma bonds because it is a little weaker. The ubiquitous nature of alkenes and their reactivity make them important to study.
Alkynes contain carbon-carbon triple bonds. They contain one sigma bond and two pi-bonds. They are sp hybridized.
The more bonds that exist between two carbon atoms, the shorter the distance is between the two carbon atoms. Another way to think about it is the greater the “s-character” of the hybridization, the closer an atom will hold its electrons to its nucleus because s-orbitals have the electron density closer to the nucleus than p-orbitals.
Percent s-character for hybrid orbitals

Hybridization and lengths of various bond types
Degrees of Unsaturation
Compared to the chemical formula for a saturated alkane, each pi bond in a compound reduces the chemical formula by two hydrogen atoms. We learned in the Mass Spectrometry chapter a formula to determine the degrees of unsaturation of a molecule from its chemical formula. Each degree of unsaturation represents a pi bond or a ring in the molecule. The formula we use to determine degrees of unsaturation is

Formula to calculate degrees of unsaturation
For alkenes, the C=C contains one pi-bond and accounts for one degree of unsaturation. For alkynes, the C≡C contains two pi-bonds and accounts for two degrees of unsaturation.
1. How many degrees of unsaturation are in the following compounds?
a) C7H12
b) C5H8O
c) C2H7N
d) C4H5Cl
e)

f)

Nomenclature
Alkenes and Alkynes
In IUPAC nomenclature, alkene names have the –ene suffix.

Examples of simple alkenes
For the IUPAC system, alkyne names have the –yne suffix. The simplest alkyne, ethyne, is more commonly called acetylene. Acetylene is a gas that is commonly used in torches and welding. Nearly everybody calls this compound acetylene. Very few people would call it “ethyne”. Common names for alkynes are named as substituted acetylenes. The groups on the alkyne (the acetylene) are named followed by the word “acetylene”.

Examples of simple alkynes
When alkynes are on the end of a molecule, with a hydrogen atom attached to the acetylene, they are called terminal alkynes. When two carbon groups are attached to the acetylene, the alkyne is called an internal alkyne.

When naming alkenes, the longest carbon chain that contains the double bond is identified. The chain is numbered starting from the side nearest the C=C. The location of the double bond is identified with the lowest carbon number of the two carbon atoms that make up the double bond. The older IUPAC system put the numbers before the name. In 1993, IUPAC made a change to put the number location of the C=C directly before the –ene suffix. Both are very commonly used, so you should learn both of them.
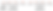
Alkynes are identified by the –yne suffix. The location of the triple bond is also identified with a number.

The longest carbon chain, the base chain, must include the alkene. In molecule below, a seven-carbon chain can be found, but the six-carbon base chain on the right is the one we use.

If a compound contains both a C=C and C≡C, the base chain should include both functional groups.

We identify two carbon-carbon double bonds in a molecule by using the suffix "diene". Three double bonds is "triene", and four is "tetraene", etc.

Like alkanes, alkenes can be named as substituents of a chain with names like methyl, ethyl, etc., alkenes can also be named as substituents. The following substituent common names should be memorized.

Common names for alkenyl groups
2. Name the following compounds.



3. Draw the following compounds.
a) 2-methyl-1-butene
b) 3-propylheptene
c) 1,5-hexadiene
d) 2-pentyne
Alkenes - Cis/Trans and E/Z
Sigma bonds can easily rotate around.

But pi-bonds cannot rotate. Since part of the pi-bond is on the top of the molecule and part is on the bottom, the molecule cannot spin or rotate.

But pi-bonds cannot rotate. Since part of the pi-bond is on the top of the molecule and part is on the bottom, the molecule cannot spin or rotate.

2-Butene can exist as a cis or trans isomer. We use cis if identical groups are on the same side and trans if they are on opposite sides.

But, how would we name the following alkene isomers? Which is cis and which is trans?

With no rotation of the pi bonds, these are obviously different compounds. But, sometimes, it is not easy to see if a molecule is cis or trans like it was for the 2-butenes. In compounds like this, we use the E-Z system. How easy!
The E-Z system
Each side of the double bond has two groups attached to it. These two groups are compared and assigned a priority (1-highest or 2-lowest) using the Cahn-Ingold-Prelog system like for R & S designations. If the two highest priority groups (1) are on the same side of the double bond (like cis), the prefix “Z” (from the German: Zusammen “together”) is assigned to the name. If the groups are on opposite sides of the double bond, like trans, the prefix “E” is used (from the German: Entgegen “opposite”).

E and Z examples
For our compounds above, we would get the following names.

Let’s name the following compound.

The longest chain is 7-carbons long and contains two double bonds. We label the chain from the left to the right so the double bonds have lower numbers. With the double bonds between carbons 2-3 and 4-5, the double bonds are at locations 2 and 4. The chlorine atom is on carbon 3. We therefore have the name 3-chloro-hepta-2,4-diene.

But, we haven’t described the orientation of the double bonds. We need to describe them. Let’s look at bond-2 first. We need to remember that hydrogen atoms are not always drawn. It helps if we draw them in. We see on the left side that CH3 has higher priority than H and on the right side, Cl has higher priority than carbon. With the top priority groups on the same side, this is a “Z” double bond.
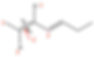
Now, let’s analyze double bond-4. On the left side, carbon has higher priority than H. On the right side, CH2 has higher priority than H. Since the top priority groups are opposite each other, this double bond is labeled “E”.

We can now give a complete name to the compound.

4. Label each of the following alkenes as cis or trans.
a)

b)
c)


d)

5. Label each of the following alkenes as E or Z.
a)
b)


c)
d)


6. Draw the following alkenes.
a) 2-chloro-3-methyl-2Z-pentene
b) cis-2-pentene
c) trans-1,2-dibromoethene
d) 1-chloro-1-fluoro-(1E,3Z)-pentadiene
7. Name the following alkenes. Remember to use cis/trans or E/Z as appropriate.
a)

b)
c)

d)

Answers
1. a) 2 b) 2 c) 0 d) 2 e) 4 f) 3
2.

3.


4.

5.

6.




7.
a)

b)

c)


d)
