The periodic table in organic chemistry
The periodic table is intimidating to beginning chemistry students. The fact is, it is pretty important. There is no need to memorize the entire thing (although it can be done fairly easily with a few choice mnemonics). The reason it is convenient to know the placement of some of the elements on the periodic table is we can quickly get information, like electronegativity and atomic size, from some simple periodic trends.
Electronegativity
It’s always a competition. In the world of atomic bonds, it’s a tug-of-war. Two atoms are engaged in a battle for the negative electrons that are between them making the bond(s). Sometimes, the atoms are equally matched in the tug-of-war. For instance, in the hydrogen molecule, H-H, both hydrogen atoms have the same electronegativity and are equally matched so they equally share the two negative electrons in the bond. We call this a nonpolar bond. But, they are not always equally matched. Sometimes, one of the atoms is a little “stronger” and begins to win the tug-of-war. This “stronger” atom pulls the negative electrons a little closer to itself. Below, we have part of an organic compound, the carbonyl. In it, a carbon atom and an oxygen atom are in a tug-of-war over the negative electrons in the bonds between them.

It’s always a competition. In the world of atomic bonds, it’s a tug-of-war. Two atoms are engaged in a battle for the negative electrons that are between them making the bond(s). Sometimes, the atoms are equally matched in the tug-of-war. For instance, in the hydrogen molecule, H-H, both hydrogen atoms have the same electronegativity and are equally matched so they equally share the two negative electrons in the bond. We call this a nonpolar bond. But, they are not always equally matched. Sometimes, one of the atoms is a little “stronger” and begins to win the tug-of-war. This “stronger” atom pulls the negative electrons a little closer to itself. Below, we have part of an organic compound, the carbonyl. In it, a carbon atom and an oxygen atom are in a tug-of-war over the negative electrons in the bonds between them.
7. Analyze this carbonyl bond.
The atom that has a more positive charge on it is (O or C)?
Carbon
Oxygen
8. For the C=O bond below, circle the partial positive charge over the partially positive charged atom and circle the partial negative charge over the partially negatively charged atom.




The atom that has a more negative charge on it is (O or C)?
Since negative charges often attack positive charges in organic chemistry, knowing which atoms are more positive and which are more negative will help us out.

If a negatively charged molecule is going to attack C=O, which atom do you think it would attack? The answer is the same as the atom it is most attracted to. Remember the old adage, opposites attract.
9. Draw an arrow from our negative pair of electrons below to the atom in C=O it would attack.


If you drew the arrow attacking the more positive atom, C, you were correct. Opposites attract. Negatives attack positives in organic chemistry. If you understand this, you are a long way towards understanding organic chemistry. Quite often, if you don’t know how a reaction will proceed, this will bail you out. Reactions often occur at the most negative spot on one molecule with the most positive spot on another molecule. Often, it is our job to find those spots. That, my friends, is one reason why we need to understand electronegativity. Negative attacks positive. Opposites attract. Beautiful. Easy. But, which atoms are more electronegative?
There are different ways to measure electronegativity. Linus Pauling developed the most common method used. Pauling died in 1994 at the age of 93 after winning two Nobel Prizes. He won one in chemistry (1954) and one in peace (1962) for advocating the end of nuclear testing. Pauling’s scale gives the most electronegative element, fluorine (F), an electronegativity value of 4.0. All other elements are less than this.

The closer an element is to fluorine on the periodic table, the more electronegative it is. That means it is “stronger” in the tug-of-war for the negative electrons in the bond and therefore has a partial negative charge. As we go towards the upper right of the periodic table (closer to fluorine) atoms become more electronegative. See the numbers in the chart above. Hydrogen has an electronegativity of 2.2, so it is slightly less electronegative than carbon (C) and more electronegative than boron (B). Notice that most of the halogens are also more electronegative than carbon. A bond between two atoms that has one atom that is more negative and one atom that is more positive is called a polar bond. We say that atom has a dipole moment. We can easily show which atom is more negative by drawing a dipole moment arrow () above the bond. The head of the arrow points towards the more negative atom, and the plus part of the arrow is, of course, above the more positive atom.

10. Above each bond below, draw dipole moment arrows pointing the appropriate directions. Remember, the arrow points towards the atom with a greater density of negative electrons around it!
Sometimes in organic chemistry, you will encounter a situation where an atom has a negative charge on it. Some atoms hate to have a negative charge on them, while others are happier with the negative charge. One thing that dictates how happy an atom is with a negative charge is its electronegativity. More electronegative atoms are more likely to have a negative charge.
11. Rank the following ions from 1-6 (1 is the best) for how happy, or stable, the following negatively charged anions are.
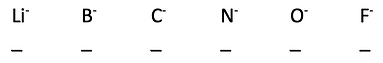
Atomic size
Electronegativity is one reason we need to know the periodic table. Another reason is the size of atoms. When a chemist is talking about the size of an atom, they are referring to the size of the electron cloud surrounding the nucleus. The general trend is atoms get smaller as you go to the right in a horizontal row of the periodic table. For instance, as we move from nitrogen to oxygen, we add one more electron to the outermost shell (the 2s) and one more proton to the nucleus. Since we added the electron to the same outer shell, we might expect oxygen to be the same size as nitrogen. But, since oxygen has one more positive proton in the nucleus, it pulls more on the negative electrons in the outermost shell than nitrogen does making it ever so slightly smaller.
As we go down a row in the periodic table, we add more positive protons to the nucleus once again. But, as we go down the periodic table, we are adding the negative electrons to entirely new shells, so the atoms are bigger. This effect is quite a bit bigger than when we go horizontally on the table. The general trend is atoms get bigger, much bigger, as we go down the periodic table.

12. Of each pair of atoms, circle the one that is bigger. Remember to have no worries!
a) C or O
b) Be or F
c) O or N
d) N or C
13. Of each pair of atoms, circle the one that is bigger.
a) Cl or Br
b) I or Cl
c) F or Cl
d) Br or F
e) P or N
f) O or S
The size of an atom can help us predict how stable it is with a negative charge on it. In nature, positive or negative charges that are localized to small areas are bad. The more we can spread out a charge on an atom, the happier the ion will be. A negative charge can spread out more over big atoms than it can on small atoms. So, the bigger the atom, the happier it would be with a negative charge.
For instance, Br- is more stable than F- because Br- is larger.
14. For each pair of anions, circle the one that is happier (or more stable).
One might think that we have two competing ideas when determining the stability of a negative ion. Br- is larger than F-, so it is more stable, but F is more electronegative than Br so one might think that F- would be more stable. In fact, we find that being a bigger atom is more important than the electronegativity of an atom when determining anion stability. It is really important to spread out the negative charge as much as we can. Nature abhors small, localized charges.
We use electronegativity when we are comparing two anions in the same horizontal row of the periodic table and we use atomic size when we are comparing two anions in the same vertical column of the periodic table.
15. For each pair of anions, circle the one that is happier (or more stable).

